MOFA+ analysis - sample level, GM with layer PCs, final metadata
Will Macnair
Neurogenomics, Neuroscience and Rare Diseases, RocheNovember 25, 2021
Last updated: 2021-11-25
Checks: 5 2
Knit directory: MS_lesions/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
The global environment had objects present when the code in the R Markdown file was run. These objects can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment. Use wflow_publish or wflow_build to ensure that the code is always run in an empty environment.
The following objects were defined in the global environment when these results were created:
| Name | Class | Size |
|---|---|---|
| q | function | 1008 bytes |
The command set.seed(20210118) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 74b907e. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rprofile
Ignored: .Rproj.user/
Ignored: ._MS_lesions.sublime-project
Ignored: .log/
Ignored: MS_lesions.sublime-project
Ignored: MS_lesions.sublime-workspace
Ignored: analysis/.__site.yml
Ignored: analysis/fig_muscat_cache/
Ignored: analysis/figure/
Ignored: analysis/ms02_doublet_id_cache/
Ignored: analysis/ms03_SampleQC_cache/
Ignored: analysis/ms04_conos_cache/
Ignored: analysis/ms05_splitting_cache/
Ignored: analysis/ms06_sccaf_cache/
Ignored: analysis/ms07_soup_cache/
Ignored: analysis/ms08_modules_cache/
Ignored: analysis/ms08_modules_pseudobulk_cache/
Ignored: analysis/ms09_ancombc_cache/
Ignored: analysis/ms09_ancombc_clean_1e3_cache/
Ignored: analysis/ms09_ancombc_clean_2e3_cache/
Ignored: analysis/ms09_ancombc_mixed_cache/
Ignored: analysis/ms10_muscat_run01_cache/
Ignored: analysis/ms10_muscat_run02_cache/
Ignored: analysis/ms10_muscat_template_broad_cache/
Ignored: analysis/ms10_muscat_template_fine_cache/
Ignored: analysis/ms11_paga_cache/
Ignored: analysis/ms12_markers_cache/
Ignored: analysis/ms13_labelling_cache/
Ignored: analysis/ms14_lesions_cache/
Ignored: analysis/ms15_mofa_sample_gm_cache/
Ignored: analysis/ms15_mofa_sample_gm_final_meta_cache/
Ignored: analysis/ms15_mofa_sample_gm_superclean_cache/
Ignored: analysis/ms15_mofa_sample_wm_cache/
Ignored: analysis/ms15_mofa_sample_wm_final_meta_cache/
Ignored: analysis/ms15_mofa_sample_wm_new_meta_cache/
Ignored: analysis/ms15_mofa_sample_wm_superclean_cache/
Ignored: analysis/ms15_patients_cache/
Ignored: analysis/ms15_patients_gm_cache/
Ignored: analysis/ms15_patients_sample_level_cache/
Ignored: analysis/ms15_patients_w_ms_cache/
Ignored: analysis/supp06_sccaf_cache/
Ignored: analysis/supp07_superclean_check_cache/
Ignored: analysis/supp09_ancombc_cache/
Ignored: analysis/supp09_ancombc_mixed_cache/
Ignored: analysis/supp09_ancombc_superclean_cache/
Ignored: analysis/supp10_muscat_cache/
Ignored: analysis/supp10_muscat_ctrl_gm_vs_wm_cache/
Ignored: analysis/supp10_muscat_gm_layers_effects_cache/
Ignored: analysis/supp10_muscat_heatmaps_cache/
Ignored: analysis/supp10_muscat_olg_pc1_cache/
Ignored: analysis/supp10_muscat_olg_pc2_cache/
Ignored: analysis/supp10_muscat_olg_pc_cache/
Ignored: analysis/supp10_muscat_regression_cache/
Ignored: analysis/supp10_muscat_soup_cache/
Ignored: analysis/supp10_muscat_soup_mito_cache/
Ignored: code/._ms10_muscat_fns_recover.R
Ignored: code/.recovery/
Ignored: code/jobs/._muscat_run09_2021-10-11.slurm
Ignored: code/muscat_plan.txt
Ignored: data/
Ignored: figures/
Ignored: output/
Ignored: tmp/
Untracked files:
Untracked: Rplots.pdf
Untracked: _dt = muscat:::.n_cells(pb) ->- as.data.table ->- setnames(c("broad", "sample_id", "n_cells"))
Untracked: analysis/supp09_ancombc_superclean.Rmd
Unstaged changes:
Modified: analysis/ms14_lesions.Rmd
Modified: analysis/ms15_mofa_sample_gm_w_layers_final_meta.Rmd
Modified: analysis/ms15_mofa_sample_wm_superclean.Rmd
Modified: analysis/supp07_superclean_check.Rmd
Modified: code/dev_edger_on_mofa_20210804.R
Modified: code/ms10_muscat_runs.R
Modified: code/ms14_lesions.R
Modified: code/ms15_mofa.R
Modified: code/supp10_muscat.R
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/ms15_mofa_sample_gm_w_layers_final_meta.Rmd) and HTML (docs/ms15_mofa_sample_gm_w_layers_final_meta.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 08fec96 | Macnair | 2021-11-24 | Add MOFA analysis for GM including layers |
| html | 08fec96 | Macnair | 2021-11-24 | Add MOFA analysis for GM including layers |
Setup / definitions
Libraries
Helper functions
source('code/ms00_utils.R')
source('code/ms09_ancombc.R')
source('code/ms10_muscat_runs.R')
source('code/ms15_mofa.R')Inputs
# specify what goes into muscat run
meta_f = "data/metadata/metadata_checked_assumptions_2021-10-08.xlsx"
olg_grps_f = 'data/metadata/oligo_groupings.txt'
comp_grps_f = 'output/ms09_ancombc/clr_clustering_GM_2021-10-19.txt'
labels_f = 'data/byhand_markers/validation_markers_2021-05-31.csv'
labelled_f = 'output/ms13_labelling/conos_labelled_2021-05-31.txt.gz'
pb_f = file.path(soup_dir, 'pb_gm_w_pcs_sum_broad_2021-11-12.rds')
pb_fine_f = file.path(soup_dir, 'pb_gm_w_pcs_sum_fine_2021-11-12.rds')
soup_f = 'data/ambient/ambient.100UMI.txt'
# define run to load
run_tag = 'run23'
time_stamp = '2021-11-15'
# define files
model_dir = file.path('output/ms10_muscat', run_tag)
muscat_f = '%s/muscat_res_dt_%s_%s.txt.gz' %>%
sprintf(model_dir, run_tag, time_stamp)
anova_f = '%s/muscat_goodness_dt_%s_%s.txt.gz' %>%
sprintf(model_dir, run_tag, time_stamp)
params_f = '%s/muscat_params_%s_%s.rds' %>%
sprintf(model_dir, run_tag, time_stamp)
ranef_dt_f = sprintf('%s/muscat_ranef_dt_%s_%s.txt.gz',
model_dir, run_tag, time_stamp)
mds_sep_f = sprintf('%s/mds_sep_dt_%s_%s.txt.gz',
model_dir, run_tag, time_stamp)Outputs
# where to save
save_dir = 'output/ms15_mofa'
date_tag = '2021-11-16'
if (!dir.exists(save_dir))
dir.create(save_dir)
# parameters for gene selection
min_sd = log(1.5)
min_fc = log(1.5)
max_p = 0.01
n_factors = 5
sel_cl = c("OPCs / COPs", "Oligodendrocytes", "Astrocytes",
"Microglia", "Excitatory neurons", "Inhibitory neurons",
"Endothelial cells", "Pericytes")
fgsea_cut = 0.1
n_paths = 50
n_cores = 8
# parameters for plotting
min_var = 5
# output files
mofa_f = sprintf('%s/mofa_%s_%s.hdf5', save_dir, run_tag, date_tag)
fgsea_pat = sprintf('%s/mofa_fgsea_%s_%s_%s.txt',
save_dir, run_tag, '%s', date_tag)
interesting_f = sprintf('%s/mofa_interesting_genes_%s_%s.xlsx',
save_dir, run_tag, date_tag)
# what to use to illustrate random effects concept?
example_cl = "Astrocytes"
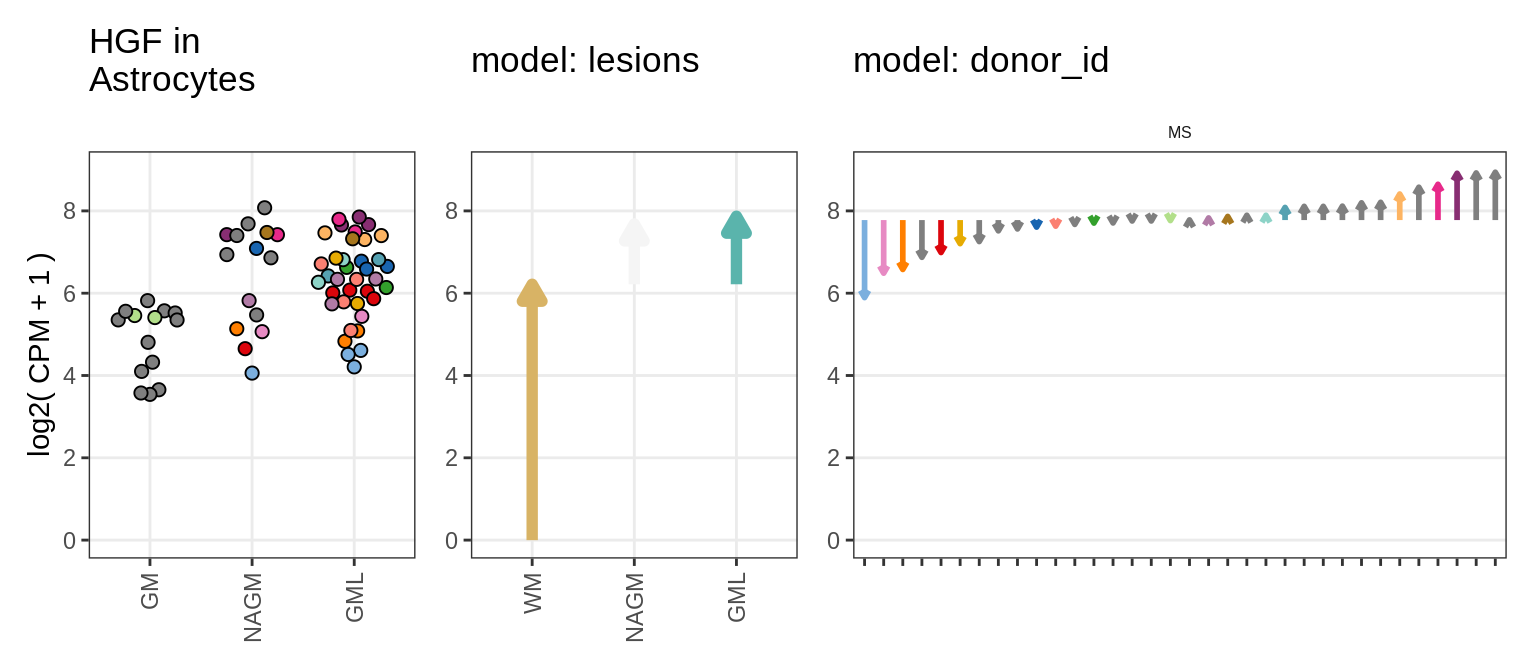
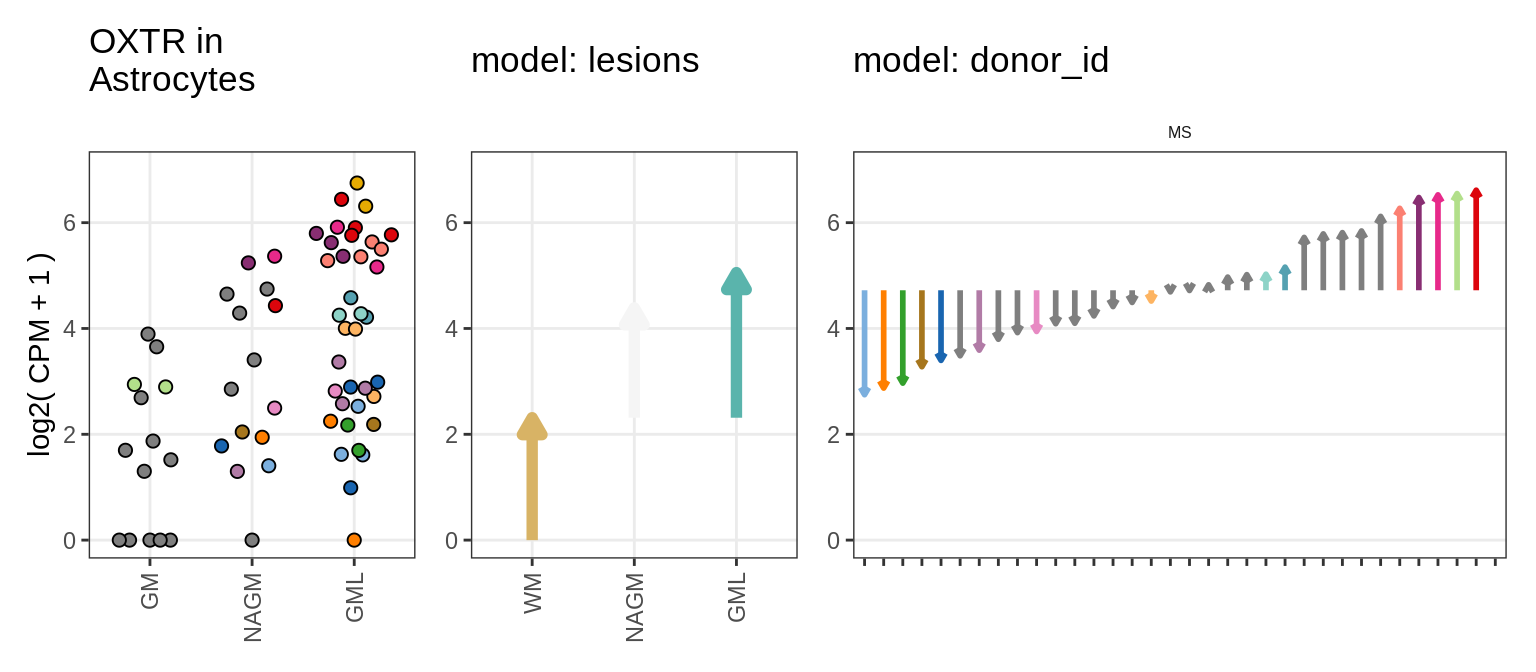
example_gs = c("HGF_ENSG00000019991", "OXTR_ENSG00000180914")Load inputs
# load parameters
params = params_f %>% readRDS
# load pseudobulk object
pb = readRDS(params$pb_f) %>% .subset_pb(params$subset_spec) %>%
subset_pb_celltypes(sel_cl) subsetting pb object restricting to samples that meet subset criteria updating factors to remove levels no longer observed# check for any massive outliers
outliers_dt = calc_log_prop_outliers(pb, dev_cut = 2)the following samples have half or more of celltypes with very extreme
(2 > MADs) log proportions:
EU005, EU044ok_samples = outliers_dt[ props_ok == TRUE ]$sample_id
pb = pb[ , ok_samples ]
# load other useful things
labels_dt = .load_labels_dt(labels_f, params$cluster_var)
magma_dt = .load_magma_dt(magma_f, pb)
tfs_dt = .load_tfs_dt(tfs_f, pb)
lof_dt = .load_lof_dt(lof_f, pb)
# load annotations
annots_dt = .get_cols_dt(pb) %>%
.[, sample := sample_id ] %>% .[, group := 'single_group'] %>%
.[, .(sample, sample_id, subject_id, subject_orig, sample_source,
age_at_death, years_w_ms, diagnosis, lesion_type, sex, pmi_cat, smoker )]
# annots_dt = add_oligo_groups(annots_dt, olg_grps_f)
annots_dt = add_compositional_groups(annots_dt, comp_grps_f)
# get random effects
ranef_dt = .load_ranef_dt(ranef_dt_f, labels_dt, pb)
# get results
res_dt = muscat_f %>% fread %>%
.load_muscat_results(labels_dt, params) %>%
.[, .(cluster_id, gene_id, symbol, var_type, coef, test_var,
logCPM, mean_soup, padj = p_adj.soup, logFC)] %>%
.[ !is.na(padj) ]
# get anova results
anova_dt = .load_anova_dt(anova_f, res_dt) %>%
.[ is.na(full), full := 1 ]
# get MDS outputs
mds_sep_dt = mds_sep_f %>% fread
if (params$cluster_var == 'type_broad')
mds_sep_dt[, cluster_id := factor(cluster_id, levels = broad_ord)]# get random effects
sd_dt = ranef_dt %>% calc_ranef_melt %>% calc_sd_dt
filter_dt = calc_filter_dt(res_dt, sd_dt, pb, anova_dt,
max_p = max_p, min_sd = min_sd, min_fc = min_fc)
filtered_dt = filter_dt %>%
.[ cluster_id %in% sel_cl ] %>%
.[ is_ms == "ms" | is_pt == "pt" ]
# check what we've got
filtered_dt[, .N, by = .(cluster_id, is_ms, is_pt)] %>%
.[, total := sum( N ), by = cluster_id ] %>%
dcast.data.table(cluster_id + total ~ is_ms + is_pt, fill = 0, value.var = "N") cluster_id total ms_not ms_pt not_pt
1: OPCs / COPs 187 34 4 149
2: Oligodendrocytes 531 254 22 255
3: Astrocytes 721 176 18 527
4: Microglia 385 71 21 293
5: Excitatory neurons 952 530 19 403
6: Inhibitory neurons 597 400 4 193
7: Endothelial cells 582 117 62 403
8: Pericytes 298 135 47 116n_cells_dt = calc_n_cells_dt(pb_fine_f, annots_dt, sel_cl)soup_dt = get_soup_logcpms(soup_f, pb)Processing / calculations
message("which genes have strong layer associations? (FDR < 1%)")which genes have strong layer associations? (FDR < 1%)layer_fits = calc_layer_fits(pb, filtered_dt, sel_cl, params)
layer_fits[ fdr < 0.01 ] %>% .[ order(fdr) ] %>%
.[, .(celltype = view, pc = coef, symbol, coef = estimate %>% round(2),
log10_p = fdr %>% log10 %>% round(2)) ] %>% print celltype pc symbol coef log10_p
1: inhib ctrl_PC01 GCNT2 0.65 -5.88
2: excit ctrl_PC01 JAG1 0.79 -5.37
3: excit ctrl_PC01 ITGA4 0.76 -5.11
4: excit ctrl_PC01 CHRM2 -0.70 -4.58
5: excit ctrl_PC01 ITGA11 -0.90 -4.25
6: excit ctrl_PC01 NPFFR2 -0.97 -4.25
7: excit ctrl_PC01 GPRIN3 -0.75 -4.22
8: excit ctrl_PC01 RASGRF2 0.55 -4.22
9: excit ctrl_PC01 CDH9 0.57 -3.85
10: excit ctrl_PC01 PTGIS -0.58 -3.64
11: excit ctrl_PC01 NXPH2 -0.88 -3.59
12: excit ctrl_PC01 AC068286.1 0.49 -3.50
13: excit ctrl_PC01 LTK 0.69 -3.16
14: inhib ctrl_PC01 CD36 0.66 -3.11
15: excit ctrl_PC01 IGFBP4 0.63 -3.11
16: excit ctrl_PC01 QRFPR -0.57 -3.11
17: excit ctrl_PC01 RAB7B 0.67 -3.11
18: inhib ctrl_PC01 TMEM196 0.52 -3.11
19: excit ctrl_PC01 C10orf67 0.40 -2.88
20: excit ctrl_PC01 PDZD2 0.55 -2.85
21: excit ctrl_PC01 GALNTL6 0.56 -2.68
22: excit ctrl_PC01 STEAP3 0.42 -2.66
23: excit ctrl_PC01 TBL1X -0.51 -2.66
24: excit ctrl_PC01 TRMT9B -0.50 -2.66
25: excit ctrl_PC01 PRLR -0.55 -2.63
26: inhib ctrl_PC01 RGS12 0.42 -2.63
27: excit ctrl_PC01 CBLN2 0.58 -2.63
28: inhib ctrl_PC01 LINC02408 0.60 -2.61
29: inhib ctrl_PC01 TRIM36 0.47 -2.61
30: excit ctrl_PC01 ERG -0.60 -2.57
31: excit ctrl_PC01 AC008415.1 -0.82 -2.55
32: excit ctrl_PC01 AC010266.2 0.48 -2.55
33: inhib ctrl_PC01 SCN5A 0.49 -2.55
34: excit ctrl_PC02 LINC02232 -0.74 -2.52
35: excit ctrl_PC01 AC016687.2 -0.87 -2.50
36: excit ctrl_PC01 CLMP -0.71 -2.50
37: inhib ctrl_PC01 GNG12 0.57 -2.50
38: excit ctrl_PC01 KANK2 -0.30 -2.50
39: inhib ctrl_PC01 NR2F2 0.59 -2.50
40: excit ctrl_PC01 SOWAHA 0.49 -2.50
41: excit ctrl_PC01 DIAPH2 0.32 -2.49
42: inhib ctrl_PC01 NR2E1 0.57 -2.49
43: inhib ctrl_PC01 RXRG 0.84 -2.47
44: excit ctrl_PC01 TEK 0.63 -2.42
45: excit ctrl_PC01 U95743.1 0.51 -2.41
46: inhib ctrl_PC01 INPP4B 0.41 -2.38
47: inhib ctrl_PC01 SYT10 0.66 -2.38
48: excit ctrl_PC02 JAG1 0.55 -2.36
49: excit ctrl_PC01 LINC00390 0.52 -2.31
50: excit ctrl_PC01 NHSL2 0.56 -2.31
51: excit ctrl_PC01 ROBO3 -0.48 -2.31
52: excit ctrl_PC01 EPHA6 0.53 -2.28
53: excit ctrl_PC02 LYPD6B -0.69 -2.28
54: excit ctrl_PC01 AP001999.1 0.66 -2.28
55: excit ctrl_PC01 ZNF608 0.47 -2.28
56: excit ctrl_PC01 MAN1A1 0.44 -2.27
57: inhib ctrl_PC01 CCDC71L 0.43 -2.26
58: excit ctrl_PC01 ART3 0.45 -2.22
59: inhib ctrl_PC01 PTGFR 0.46 -2.19
60: inhib ctrl_PC01 POU3F4 0.42 -2.18
61: inhib ctrl_PC01 CALB2 0.65 -2.16
62: excit ctrl_PC01 CERKL 0.44 -2.16
63: excit ctrl_PC01 GPR83 0.67 -2.16
64: excit ctrl_PC03 LRIG3 -0.65 -2.16
65: inhib ctrl_PC01 PROX1 0.49 -2.16
66: excit ctrl_PC01 RET -0.56 -2.16
67: inhib ctrl_PC01 SYT17 0.38 -2.05
68: excit ctrl_PC01 WTIP 0.40 -2.03
69: excit ctrl_PC03 CXCL13 -0.52 -2.02
celltype pc symbol coef log10_pmofa_obj = make_mofa_obj_samples_regress_layers(pb, filtered_dt,
sel_cl, params)Creating MOFA object from a data.frame...# set up
data_opts = get_default_data_options(mofa_obj)
model_opts = get_default_model_options(mofa_obj)
train_opts = get_default_training_options(mofa_obj)
# specify how many factors
model_opts$num_factors = n_factors
# train mofa
mofa_obj = prepare_mofa(
object = mofa_obj,
data_options = data_opts,
model_options = model_opts,
training_options = train_opts
)Checking data options...Checking training options...Checking model options...model = run_mofa(mofa_obj, mofa_f)Warning: Output file output/ms15_mofa/mofa_run23_2021-11-16.hdf5 already exists, it will be replacedConnecting to the mofapy2 python package using reticulate (use_basilisk = FALSE)...
Please make sure to manually specify the right python binary when loading R with reticulate::use_python(..., force=TRUE) or the right conda environment with reticulate::use_condaenv(..., force=TRUE)
If you prefer to let us automatically install a conda environment with 'mofapy2' installed using the 'basilisk' package, please use the argument 'use_basilisk = TRUE'Warning in .quality_control(object, verbose = verbose): Factor(s) 2 are strongly correlated with the total number of expressed features for at least one of your omics. Such factors appear when there are differences in the total 'levels' between your samples, *sometimes* because of poor normalisation in the preprocessing steps.# update metadata
model = add_metadata(model, annots_dt)
# put weights and scores in MS order
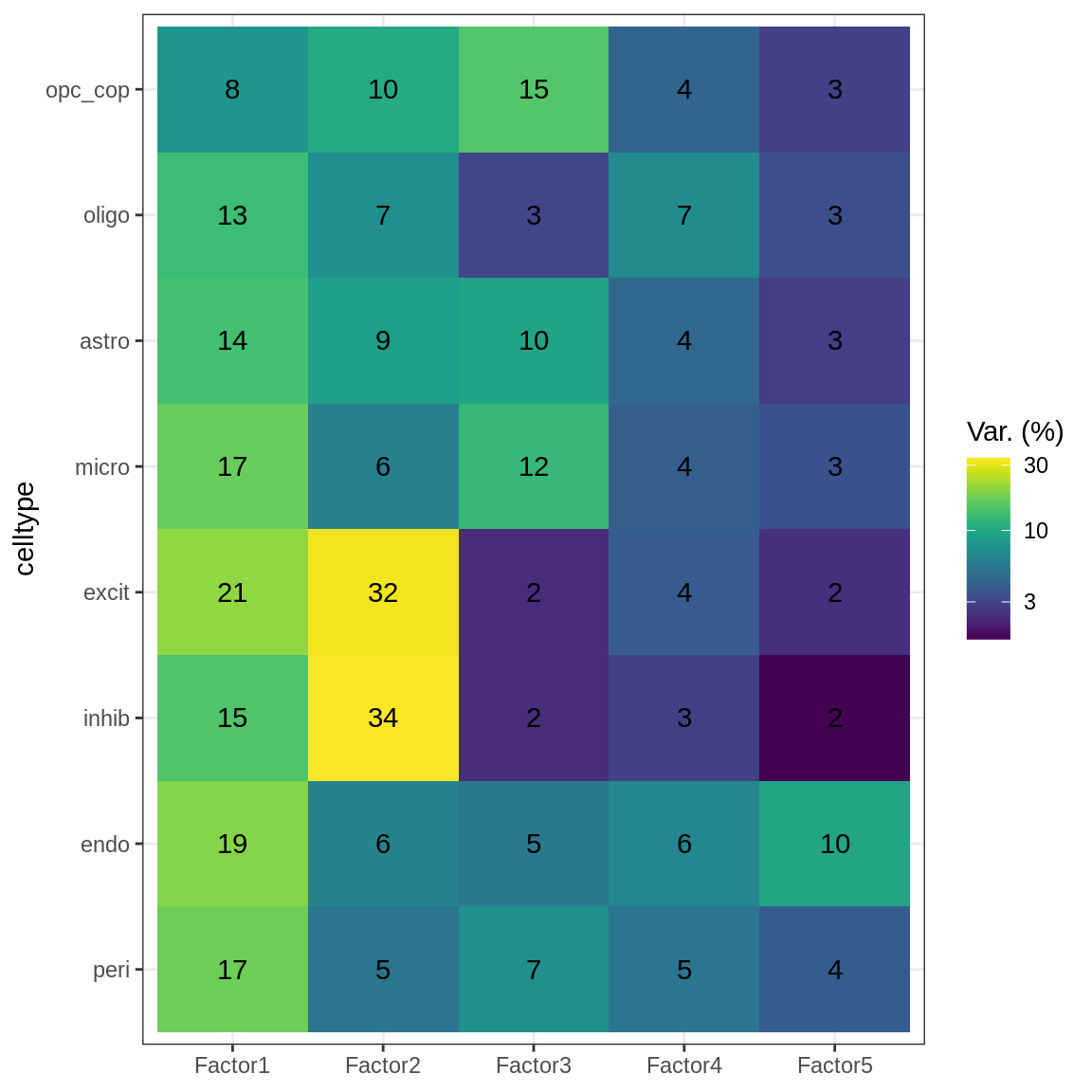
model = put_model_in_ms_order(model)var_exp_dt = get_variance_explained(model, as.data.frame = TRUE) %>%
as.data.table %>%
.[, .(
view = r2_per_factor.view %>% factor(levels = broad_short),
factor = r2_per_factor.factor,
var_exp = r2_per_factor.value
)]
to_plot_dt = var_exp_dt[ var_exp > min_var ] %>% .[order(factor, -var_exp)]w_dt = extract_weights(model, sd_dt)
fgsea_fs = sapply(names(paths_list)[1:2], function(p) sprintf(fgsea_pat, p))
if (all(file.exists(fgsea_fs))) {
# gsea_list = lapply(fgsea_fs, fread)
gsea_list = lapply(fgsea_fs, fread)
} else {
# do fgsea for these
bpparam = MulticoreParam(workers = n_cores,
progressbar = TRUE, tasks = 50)
bpstart()
gsea_list = calc_mofa_fgsea(paths_list[1:2], w_dt, fgsea_pat, fgsea_cut, bpparam)
bpstop()
}
# restrict to interesting ones
gsea_main = gsea_list %>% map( ~.x[ main_path == TRUE ]) %>% rbindlist
# show what we found
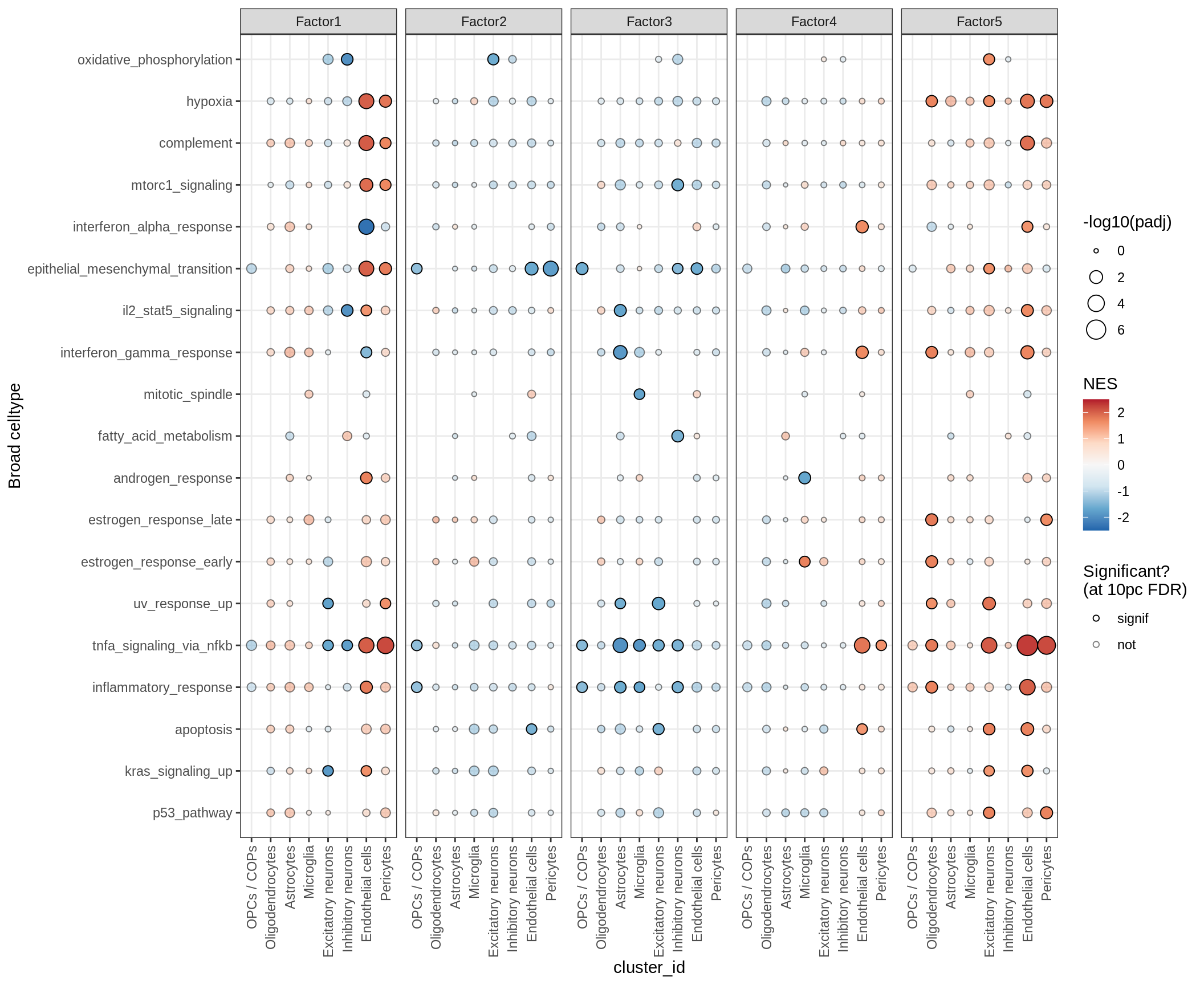
gsea_main[, .(factor, view, pathway = pathway %>% tolower %>%
str_extract("(?<=(hallmark|gobp)_).+"),
log10_p = log10(padj) %>% round(2), NES = round(NES, 2))] %>%
.[ order(factor, NES) ] %>% print factor view
1: Factor1 endo
2: Factor1 endo
3: Factor1 excit
4: Factor1 excit
5: Factor1 excit
---
302: Factor5 endo
303: Factor5 excit
304: Factor5 excit
305: Factor5 peri
306: Factor5 endo
pathway
1: interferon_alpha_response
2: response_to_type_i_interferon
3: atp_metabolic_process
4: oxidative_phosphorylation
5: proton_transmembrane_transport
---
302: inflammatory_response
303: tnfa_signaling_via_nfkb
304: negative_regulation_of_nucleobase_containing_compound_metabolic_process
305: tnfa_signaling_via_nfkb
306: tnfa_signaling_via_nfkb
log10_p NES
1: -3.23 -2.35
2: -1.79 -2.33
3: -1.97 -2.31
4: -1.93 -2.25
5: -1.75 -2.17
---
302: -3.42 2.02
303: -3.41 2.06
304: -3.81 2.17
305: -4.95 2.18
306: -7.16 2.29r2_dt = calc_r2_for_factors(model, annots_dt)Analysis
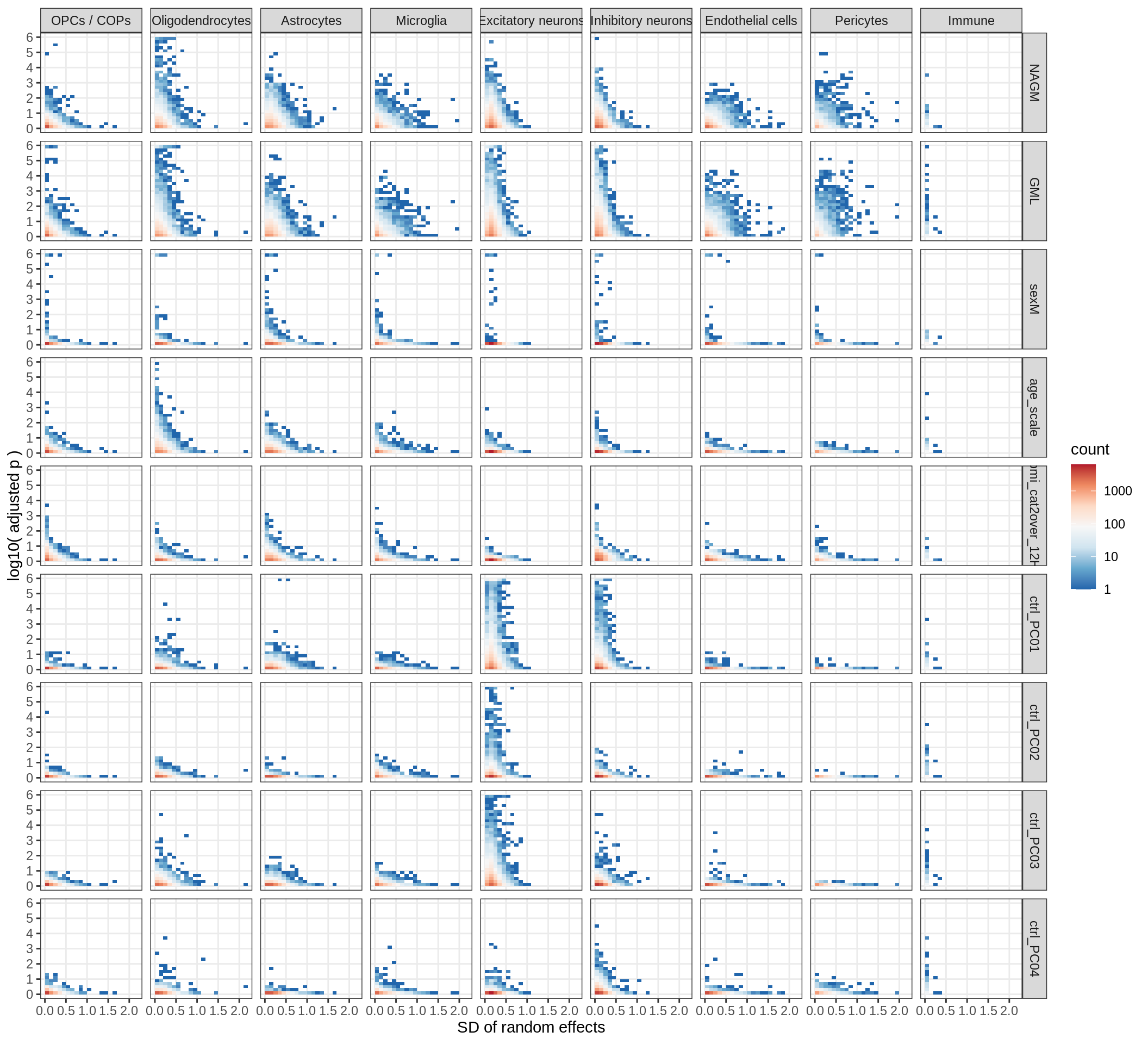
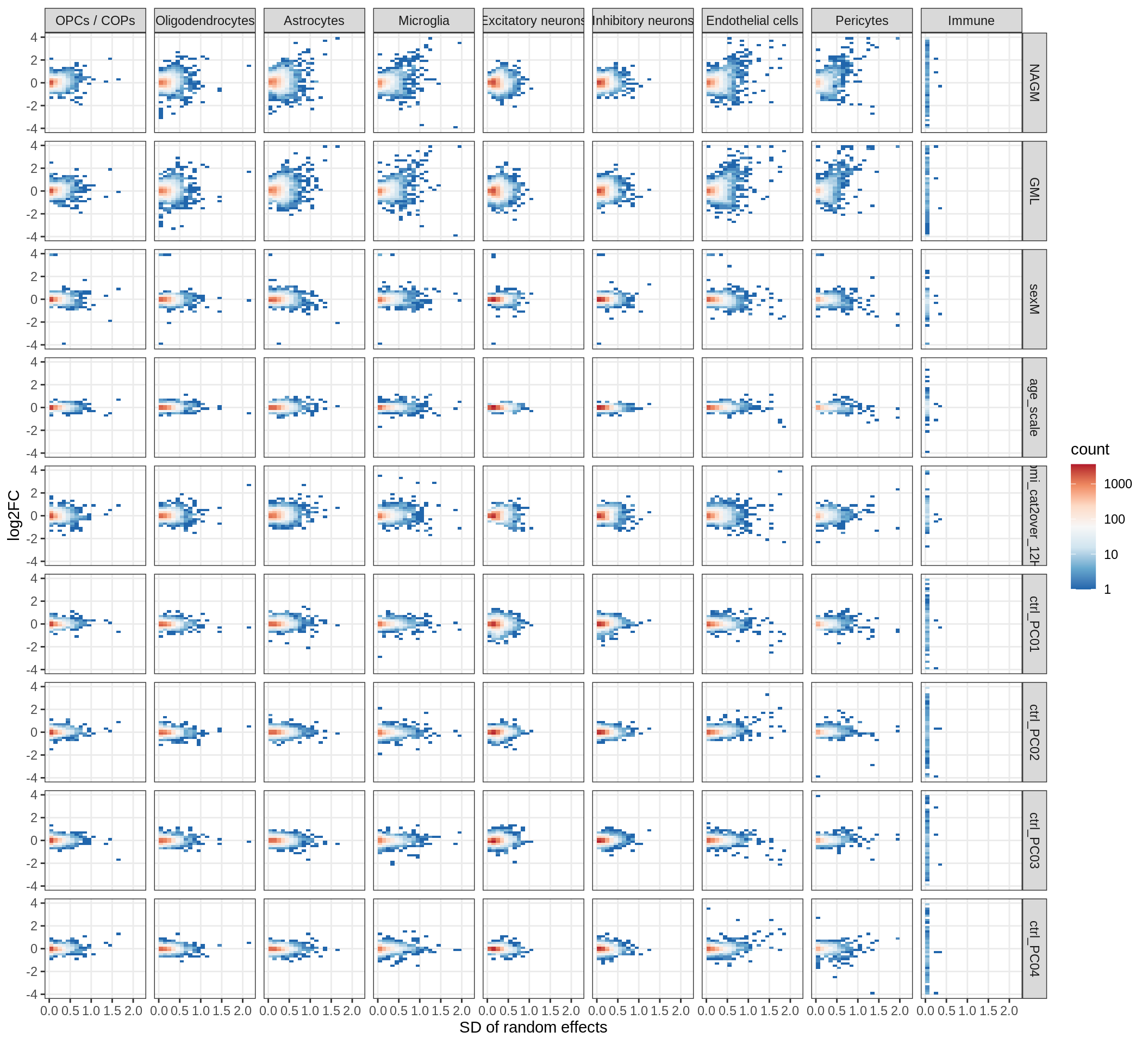
muscat results vs SD
for (what in c('log10_padj', 'log2FC')) {
cat('### ', what, '\n', sep = '')
print(plot_muscat_vs_sd(res_dt, sd_dt, NULL, what = what))
cat('\n\n')
}Cytokine effects
cyto_gs = unique(res_dt$gene_id) %>% str_subset('(^IL[0-9]+|^CCL|^CXCL|^IFN|^TGF|^TNF|^CSF)')
(plot_muscat_vs_sd_min(res_dt[ gene_id %in% cyto_gs ], sd_dt[ gene_id %in% cyto_gs ],
sel_cl, min_sd, max_p, do_labels = TRUE))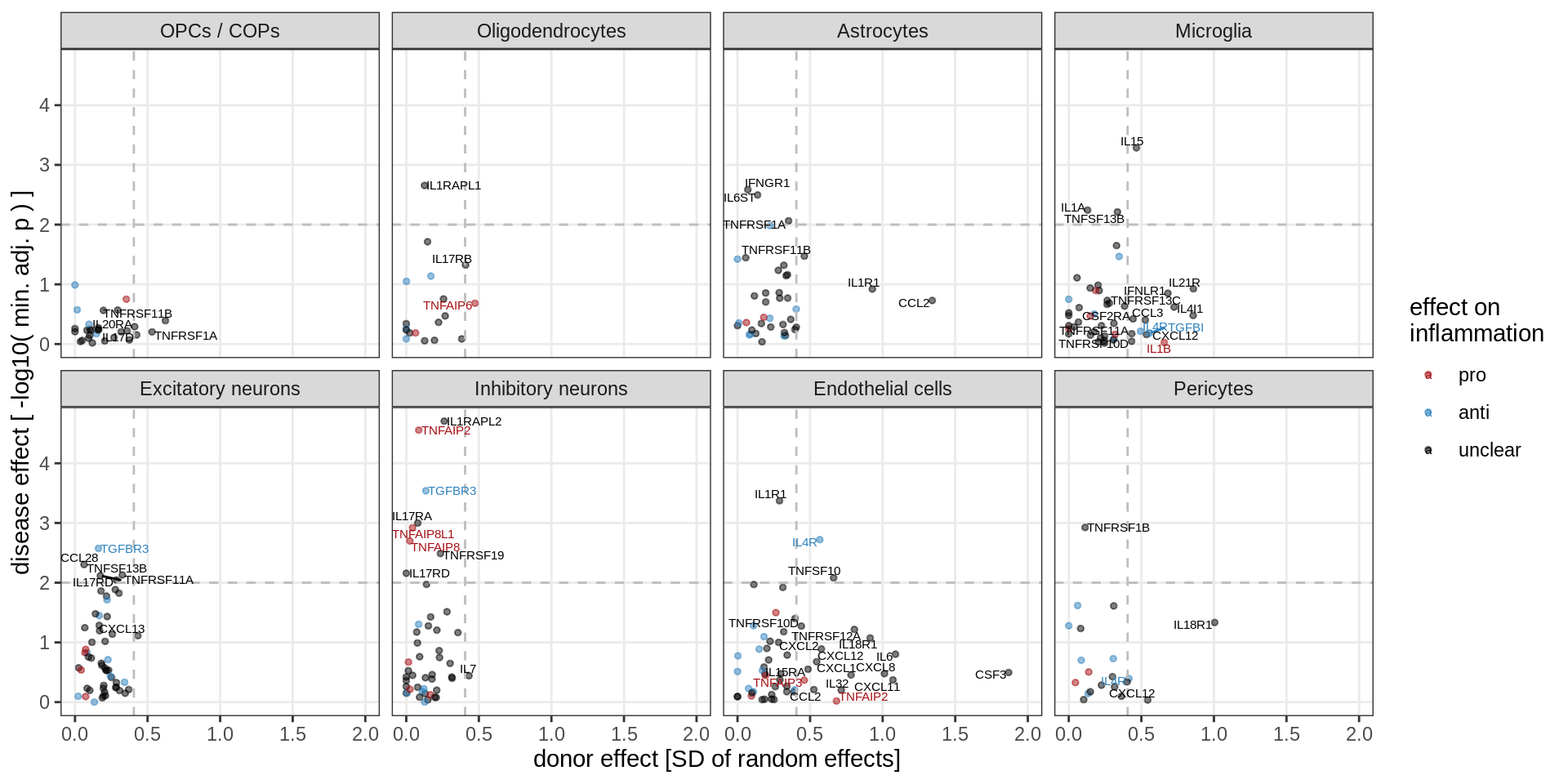
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
Ages vs duration of MS
(plot_age_duration(annots_dt))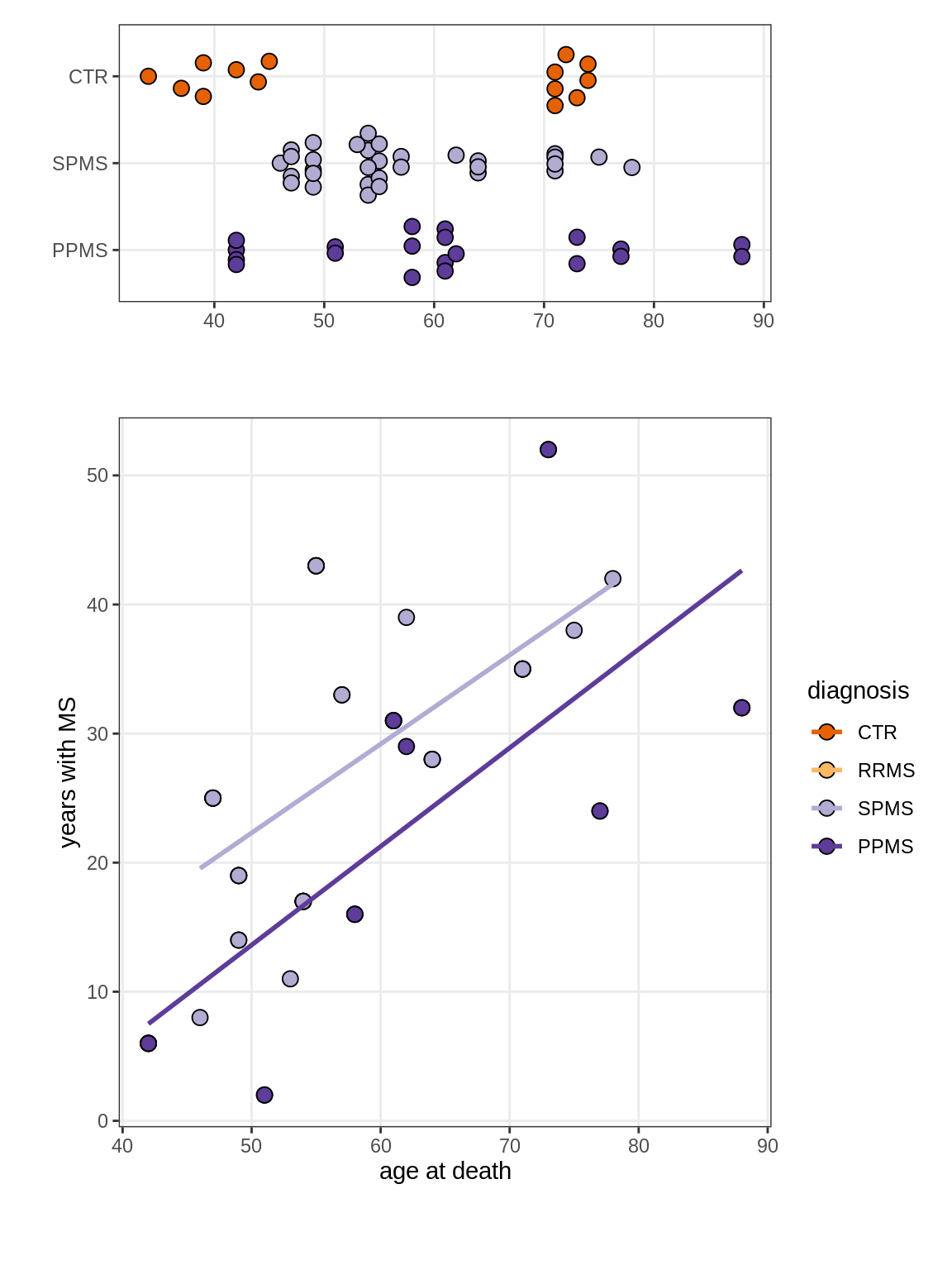
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
Data overview
(plot_data_overview(mofa_obj))Warning: `guides(<scale> = FALSE)` is deprecated. Please use `guides(<scale> =
"none")` instead.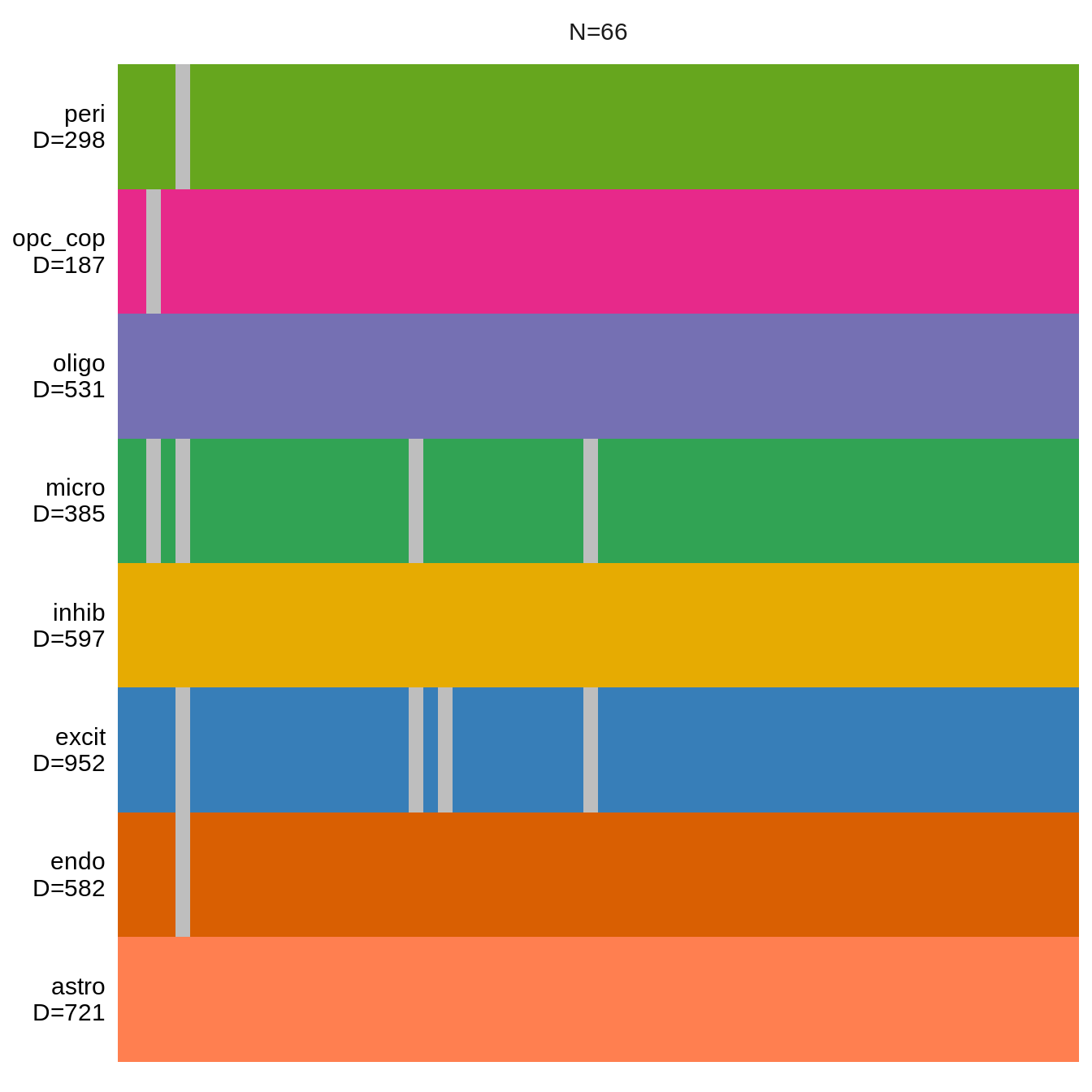
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
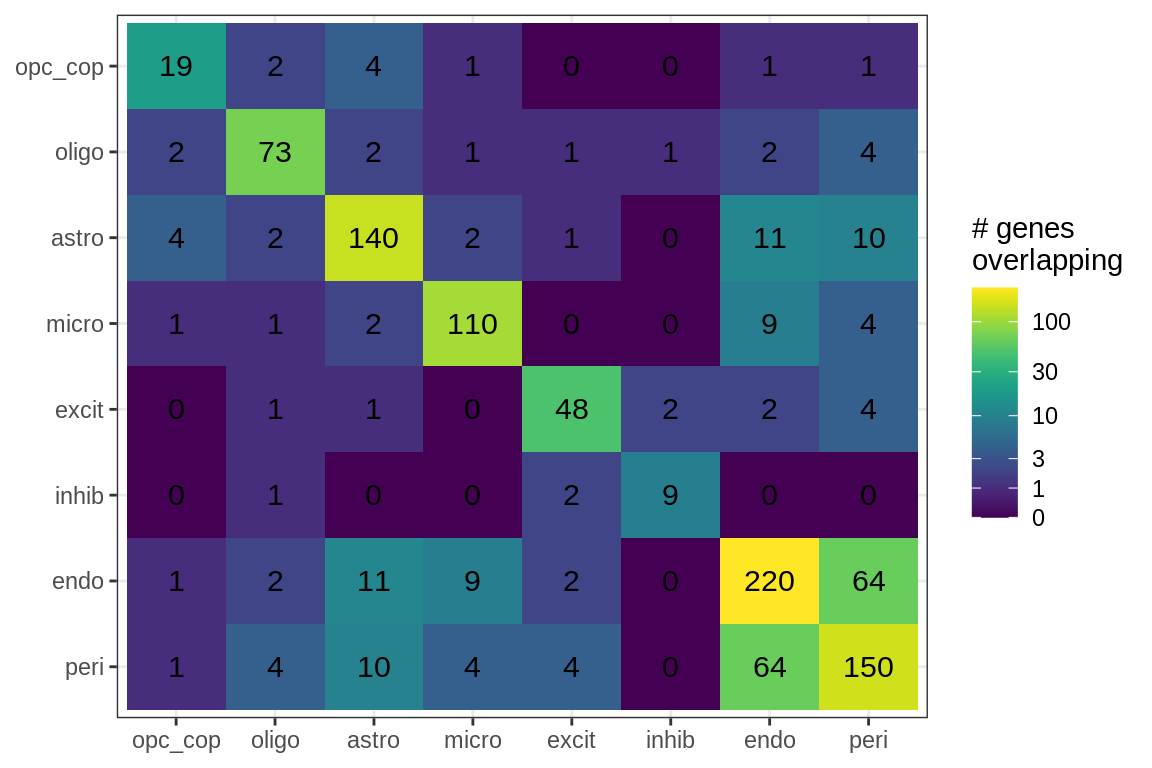
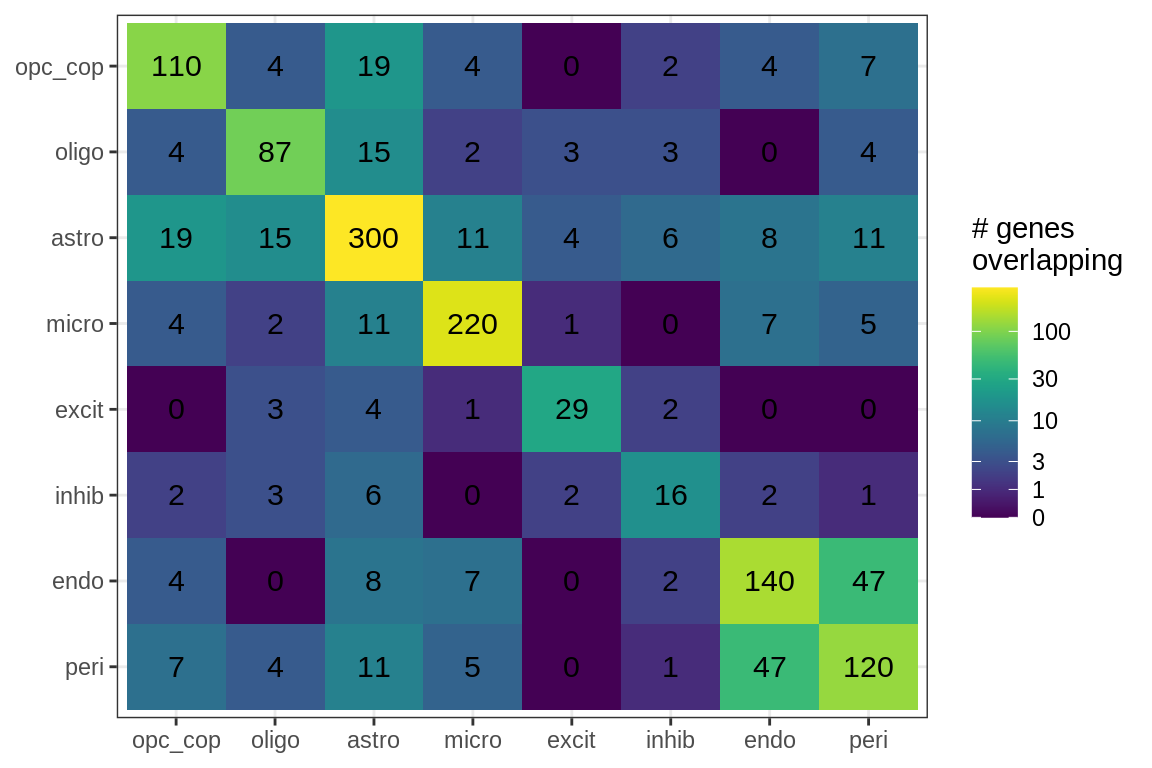
Overlapping genes
cat('### All genes\n')All genes
print(plot_gene_overlap(model))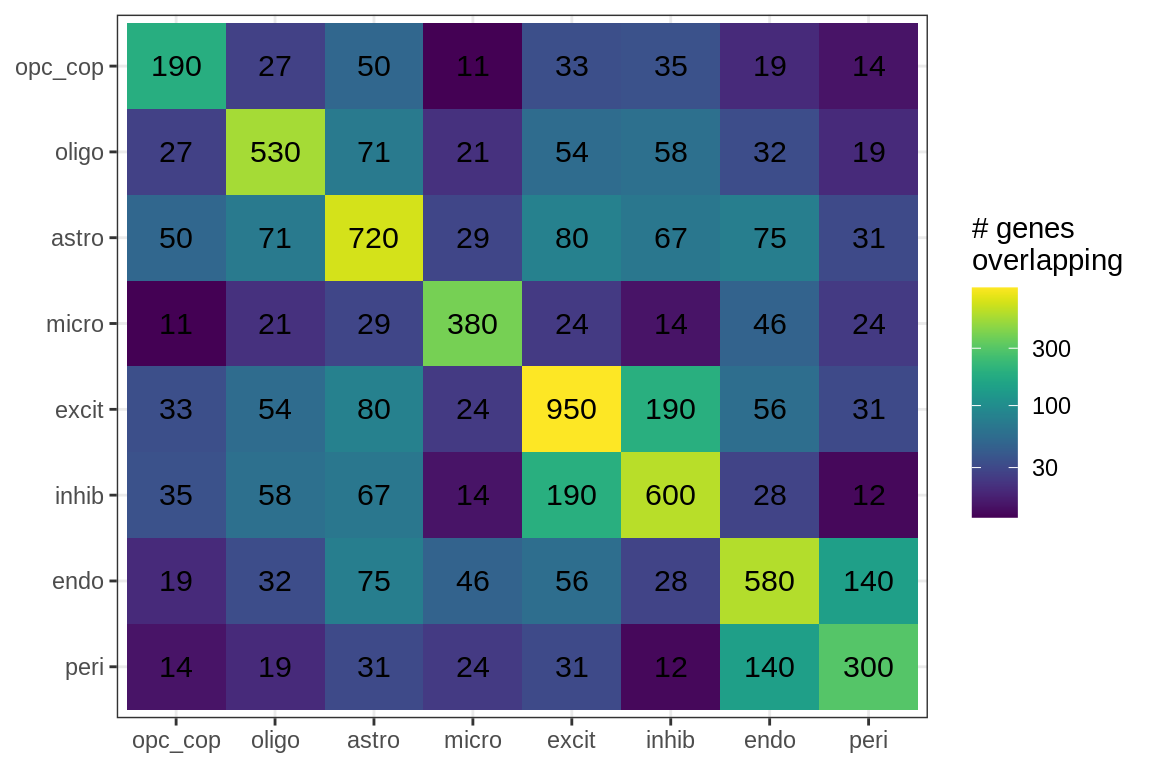
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
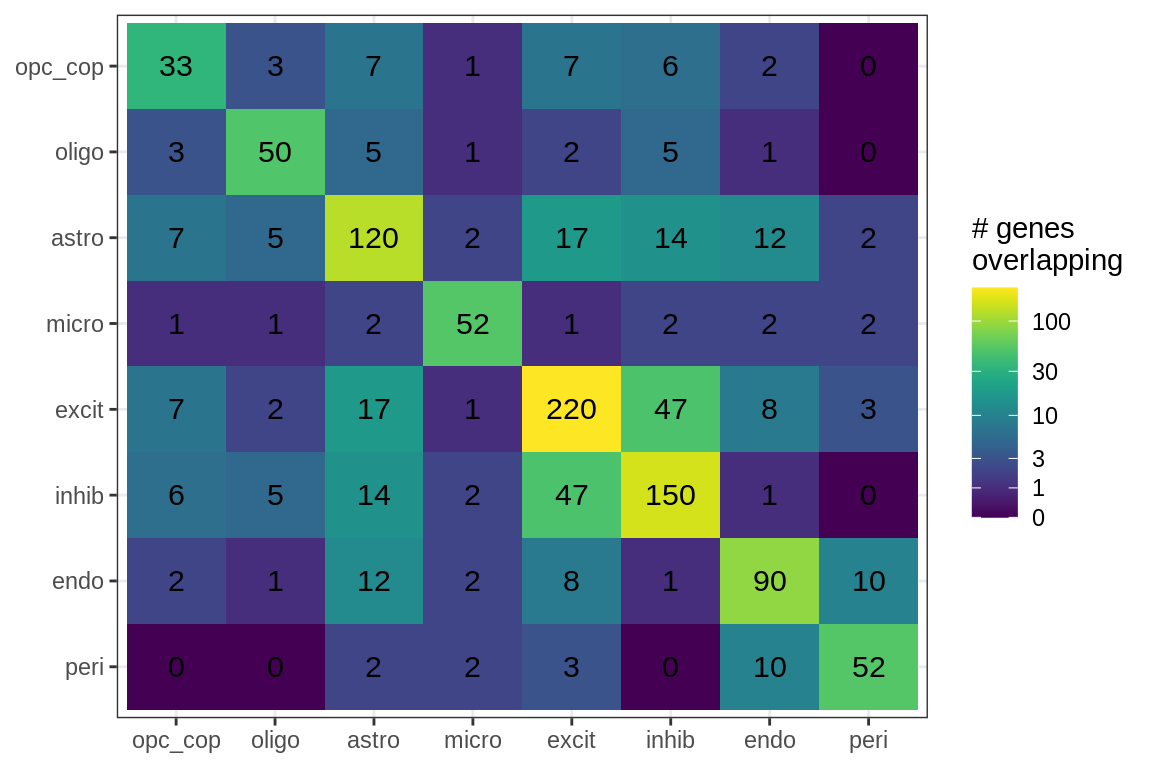
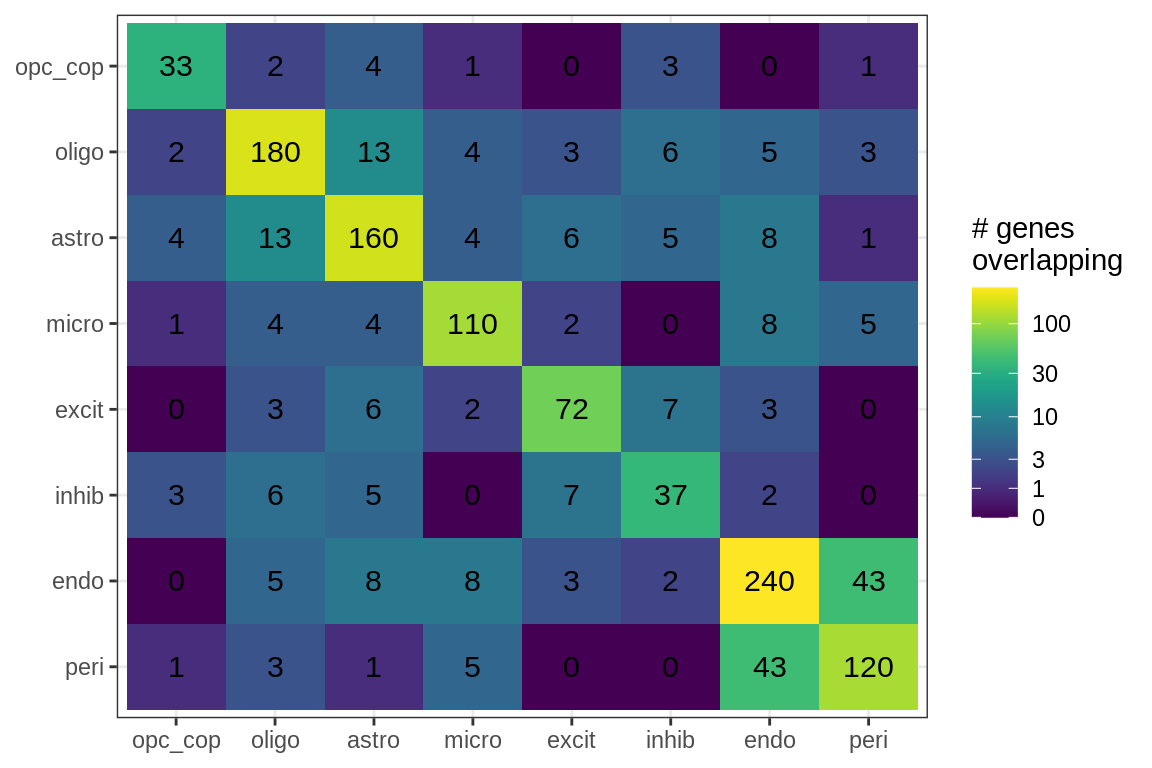
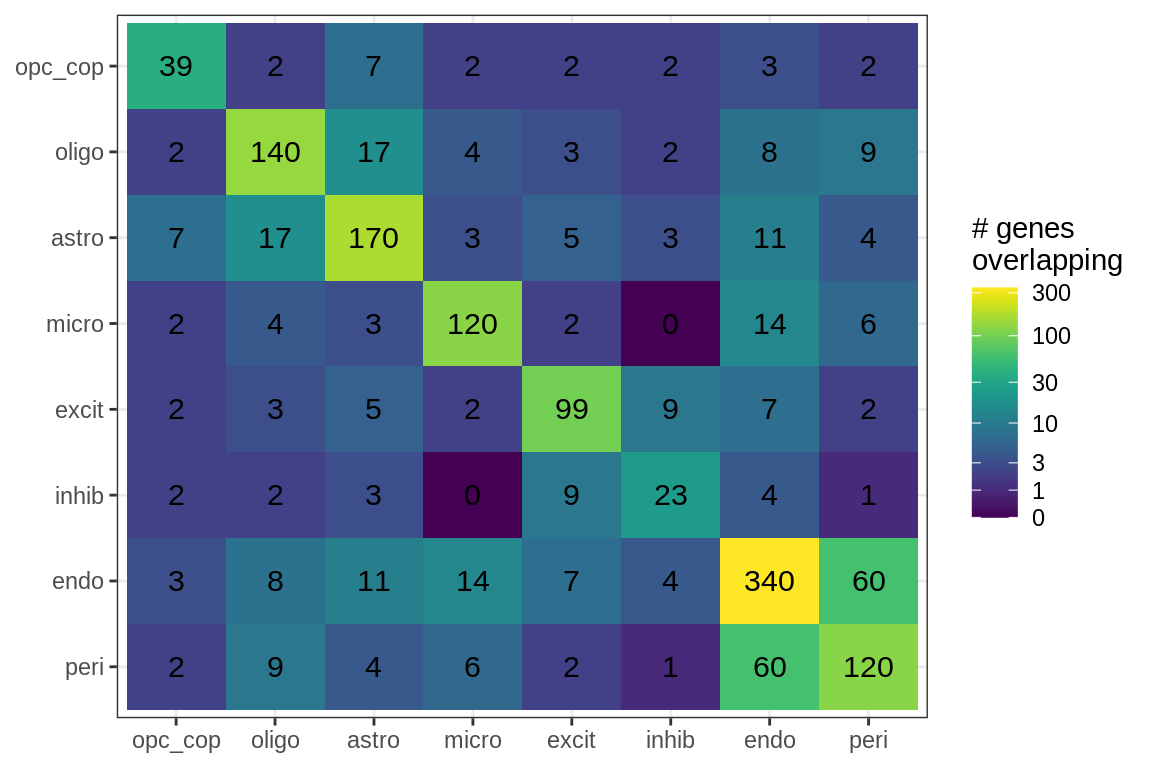
cat('\n\n')for (sel_f in factors_names(model)) {
cat('### Genes in ', sel_f, '\n', sep = '')
suppressWarnings(print(plot_gene_overlap(model, sel_f = sel_f, w_cut = 0.2)))
cat('\n\n')
}Factor distributions
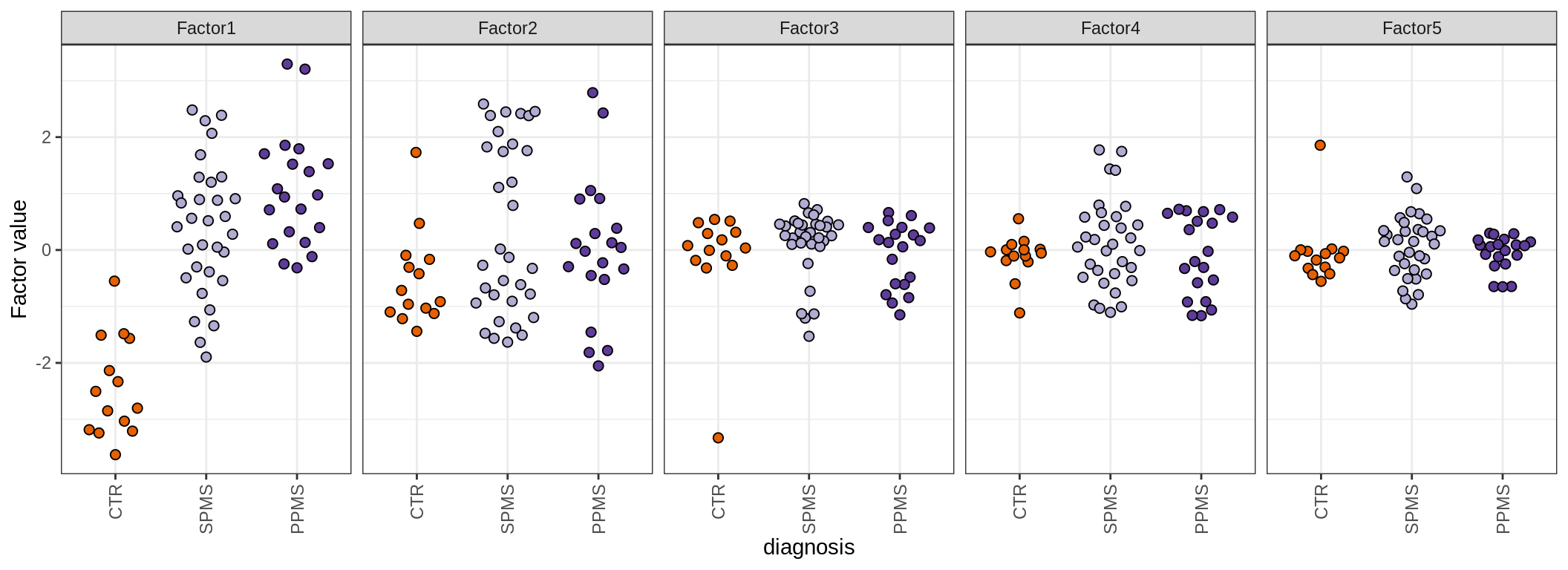
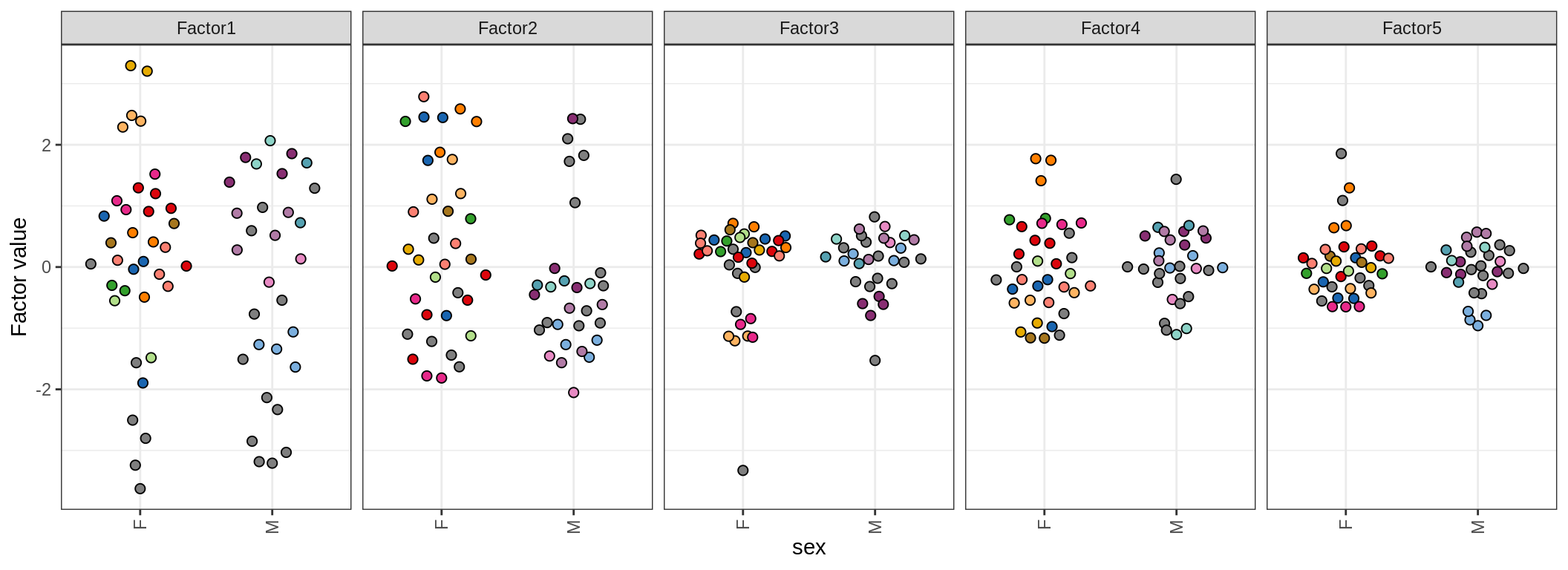
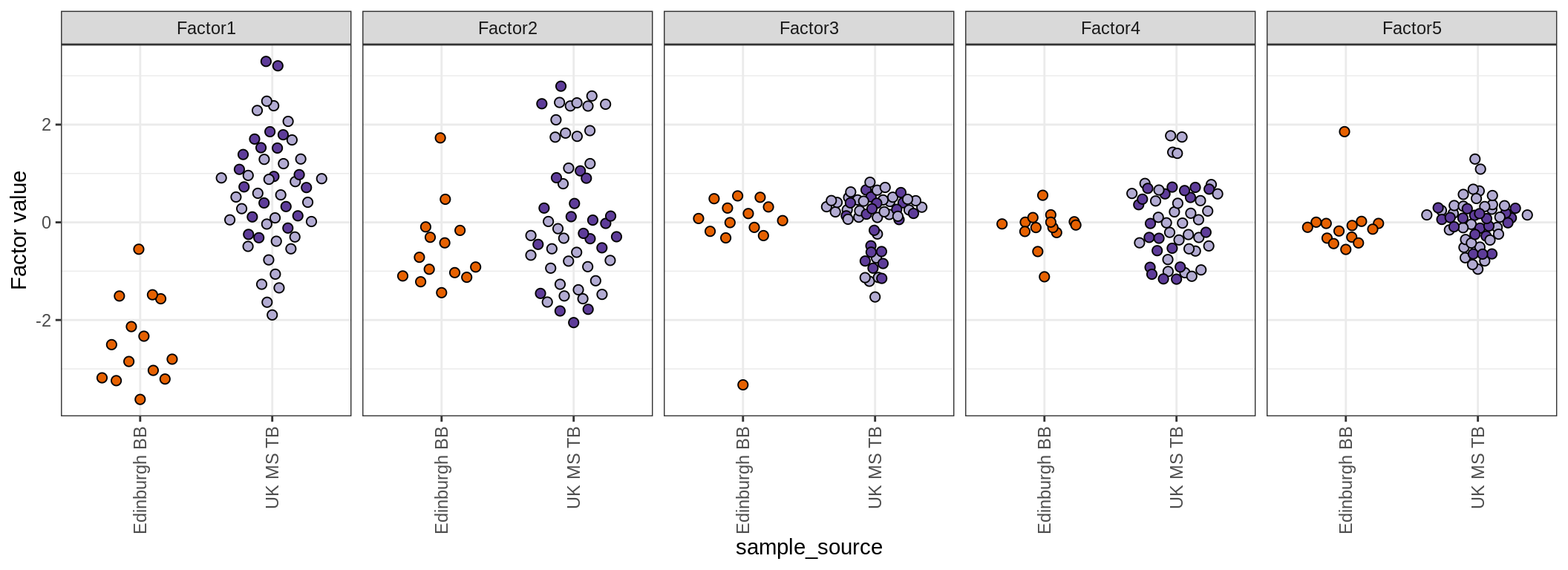
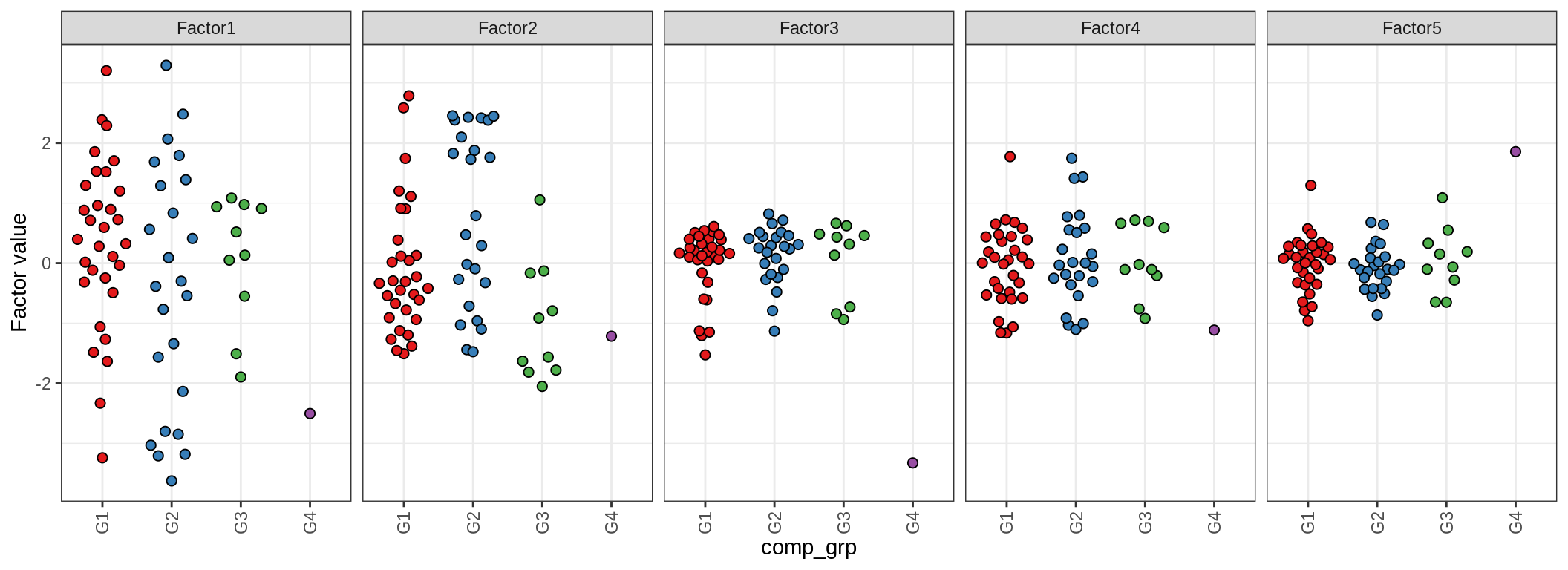
for (annot in c('lesion_type', 'diagnosis', 'sex', 'sample_source', 'smoker', 'comp_grp')) {
cat('### by ', annot, '\n', sep = '')
print(plot_factors_univariate(model, annots_dt, pb, by = annot))
cat('\n\n')
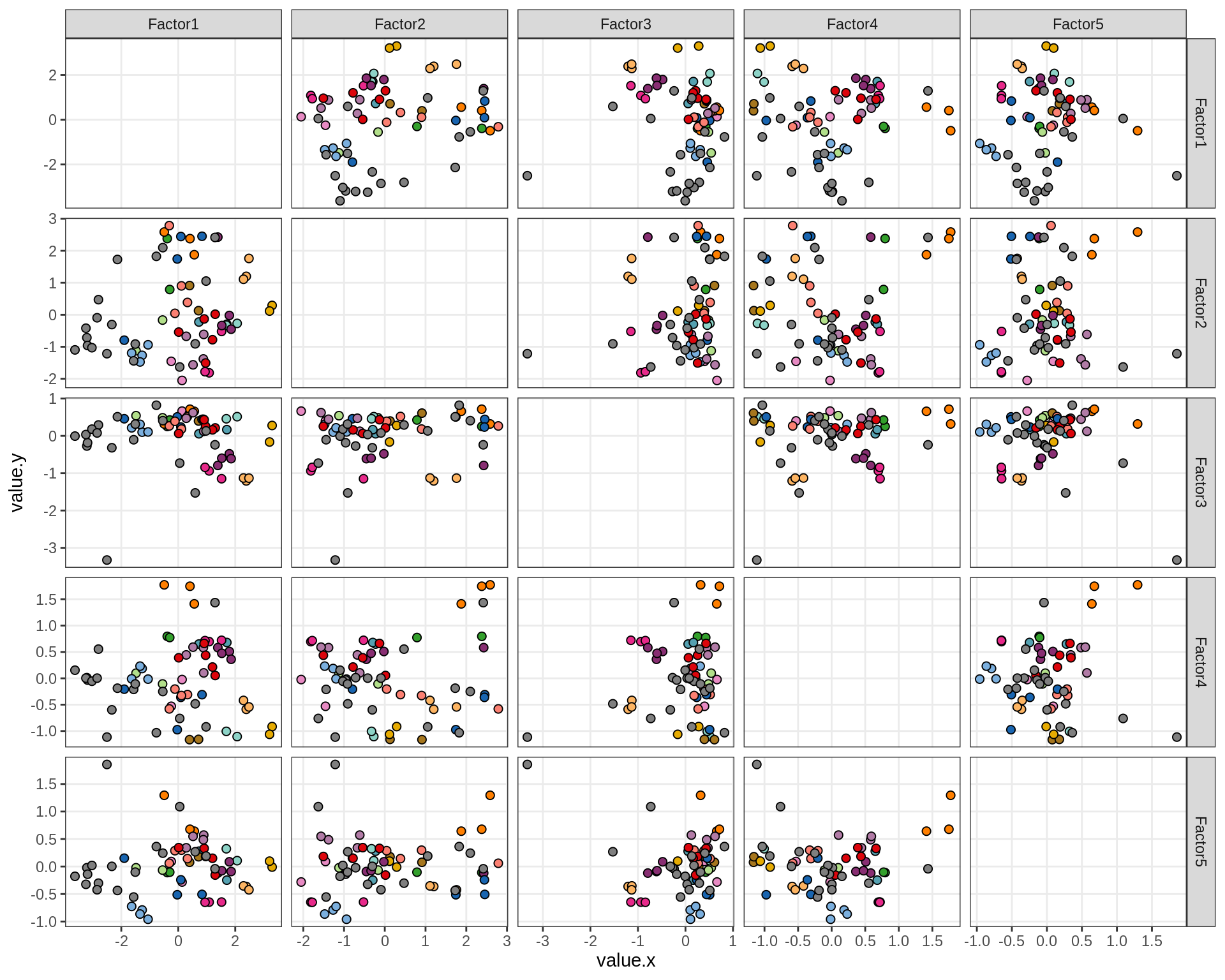
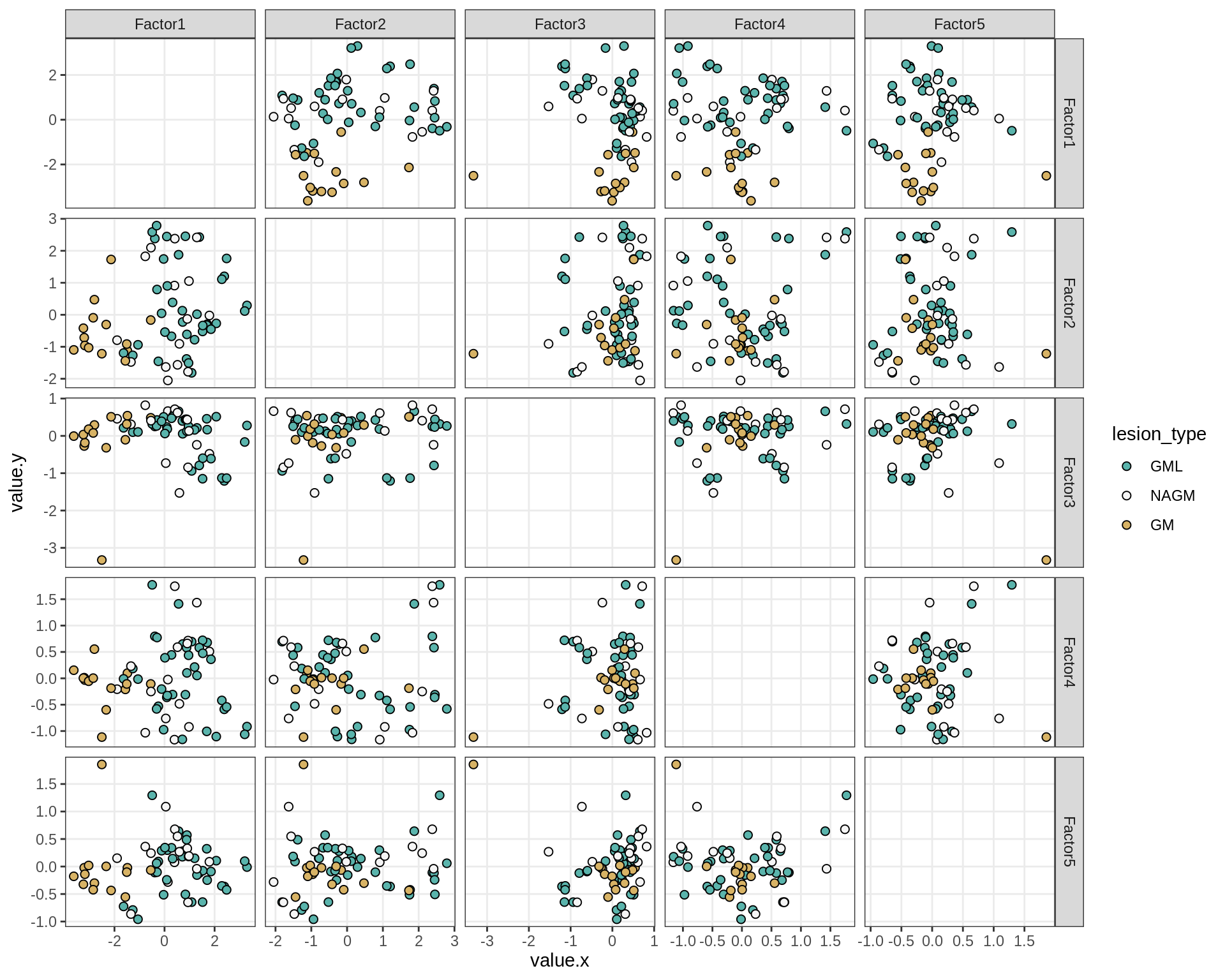
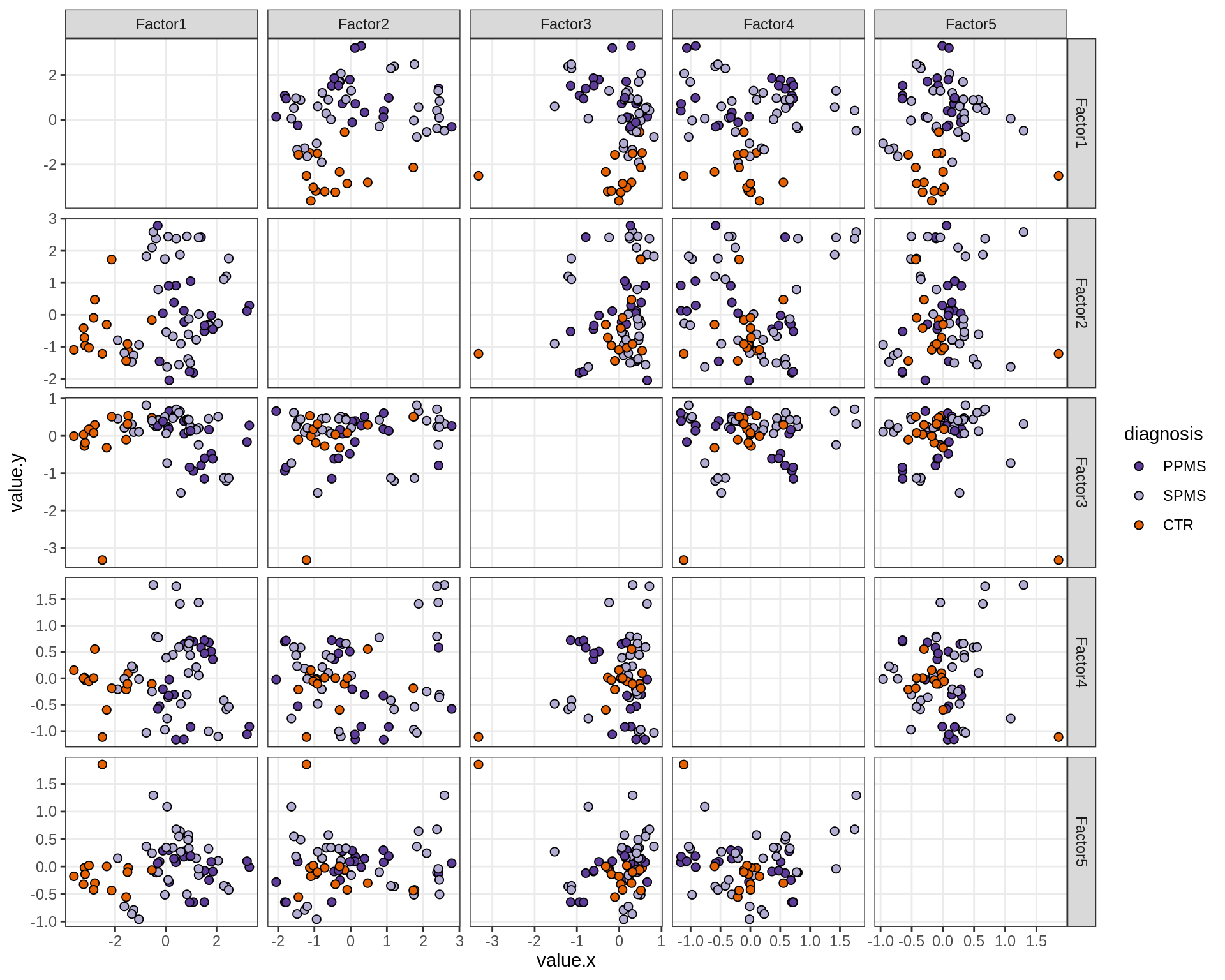
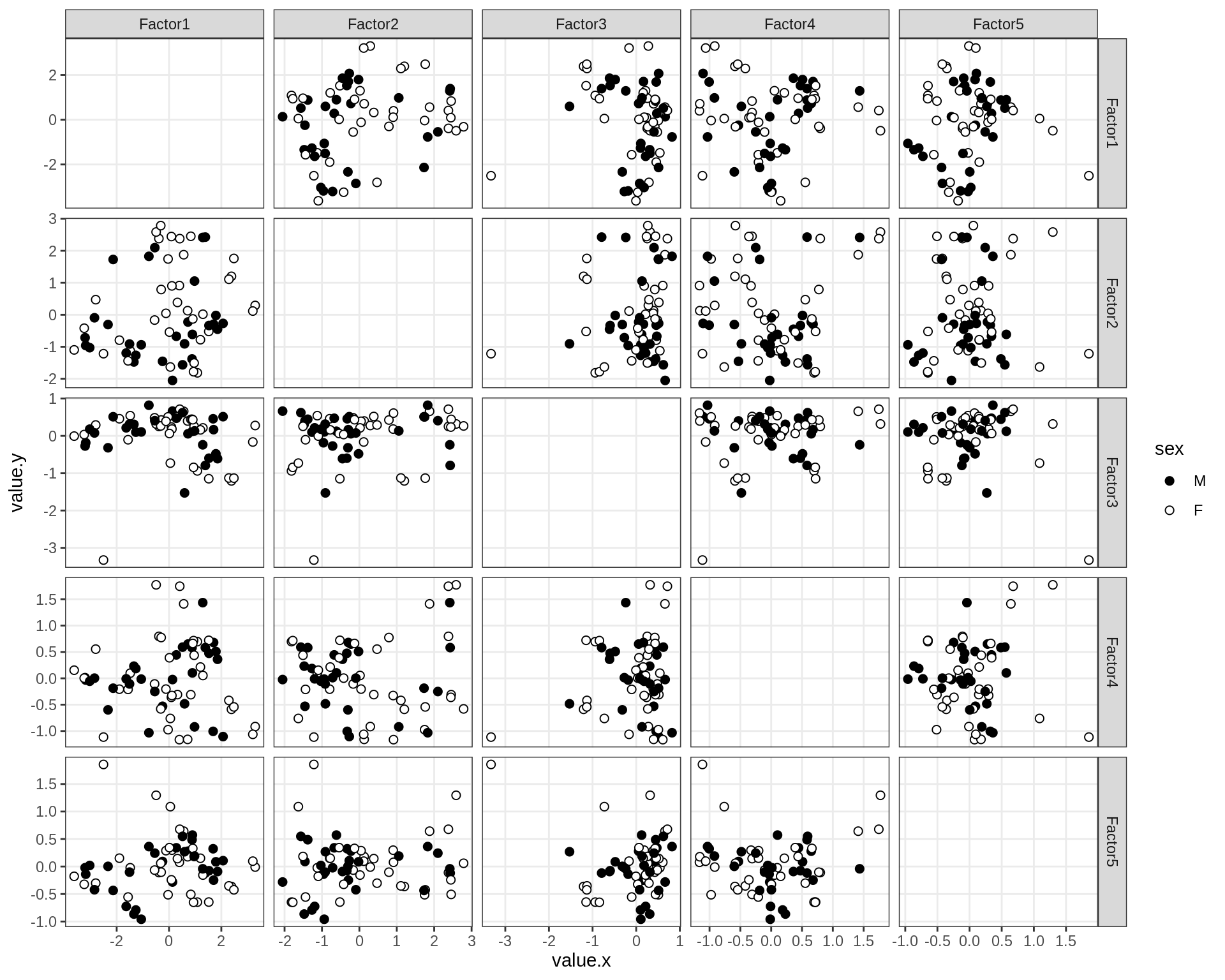
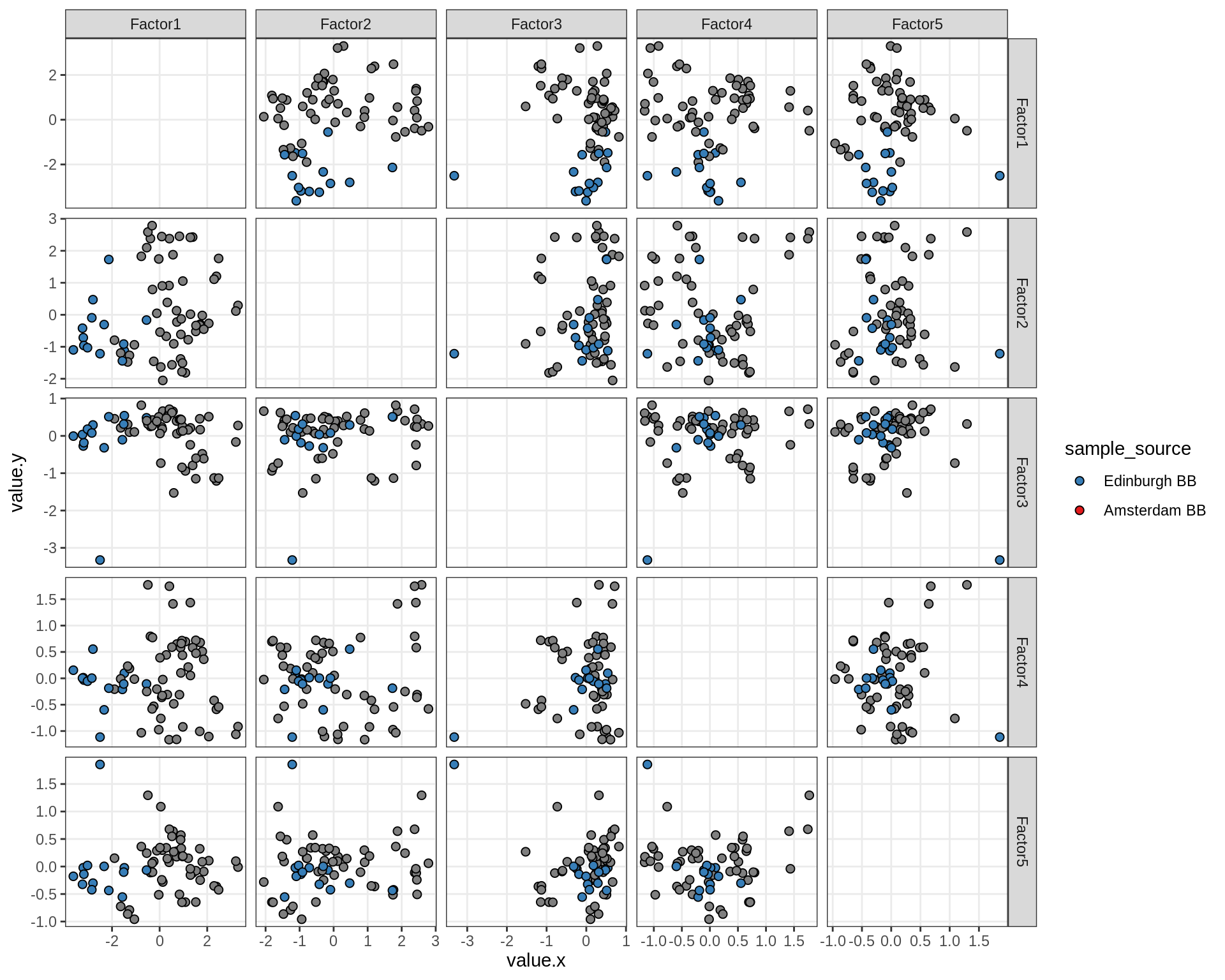
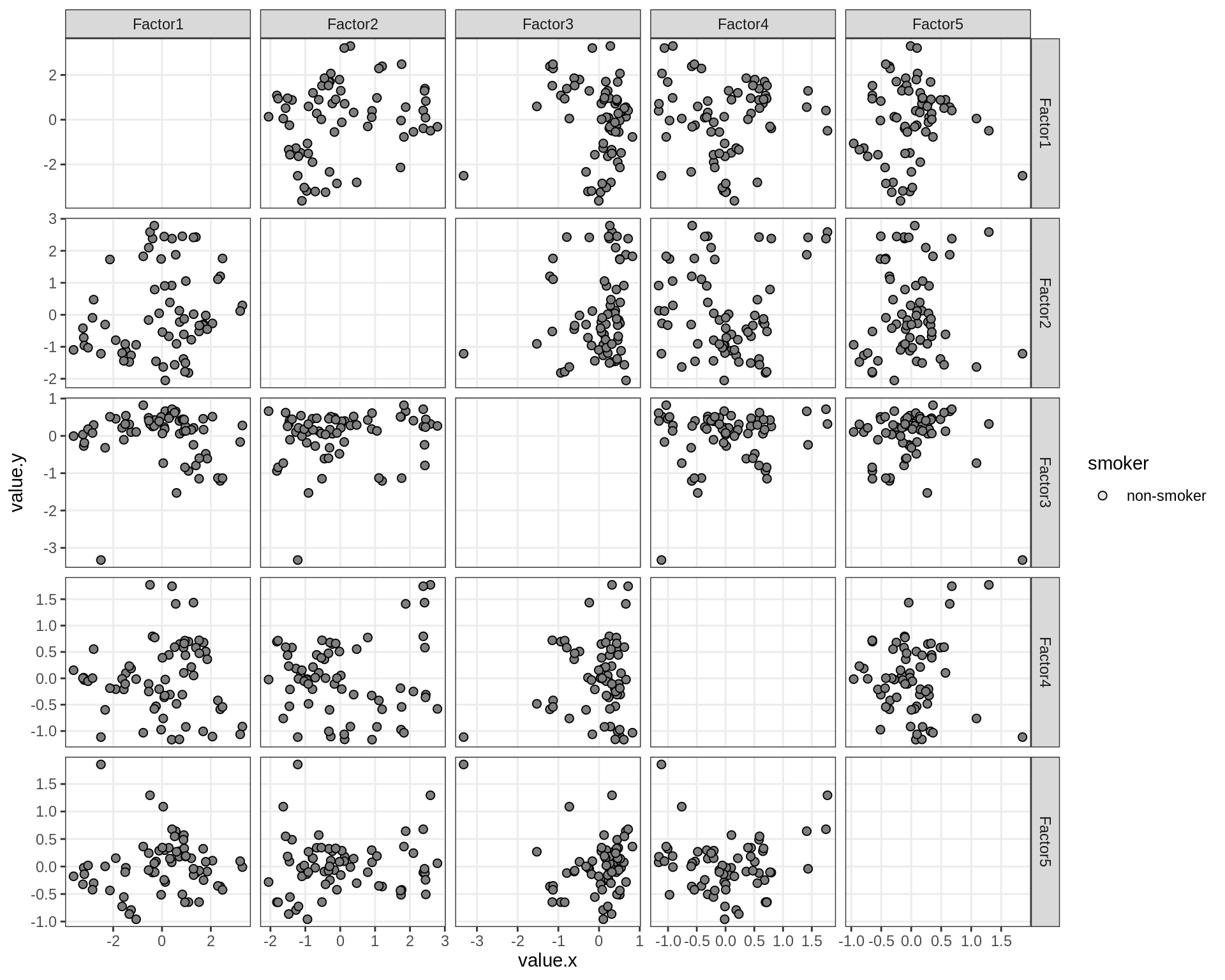
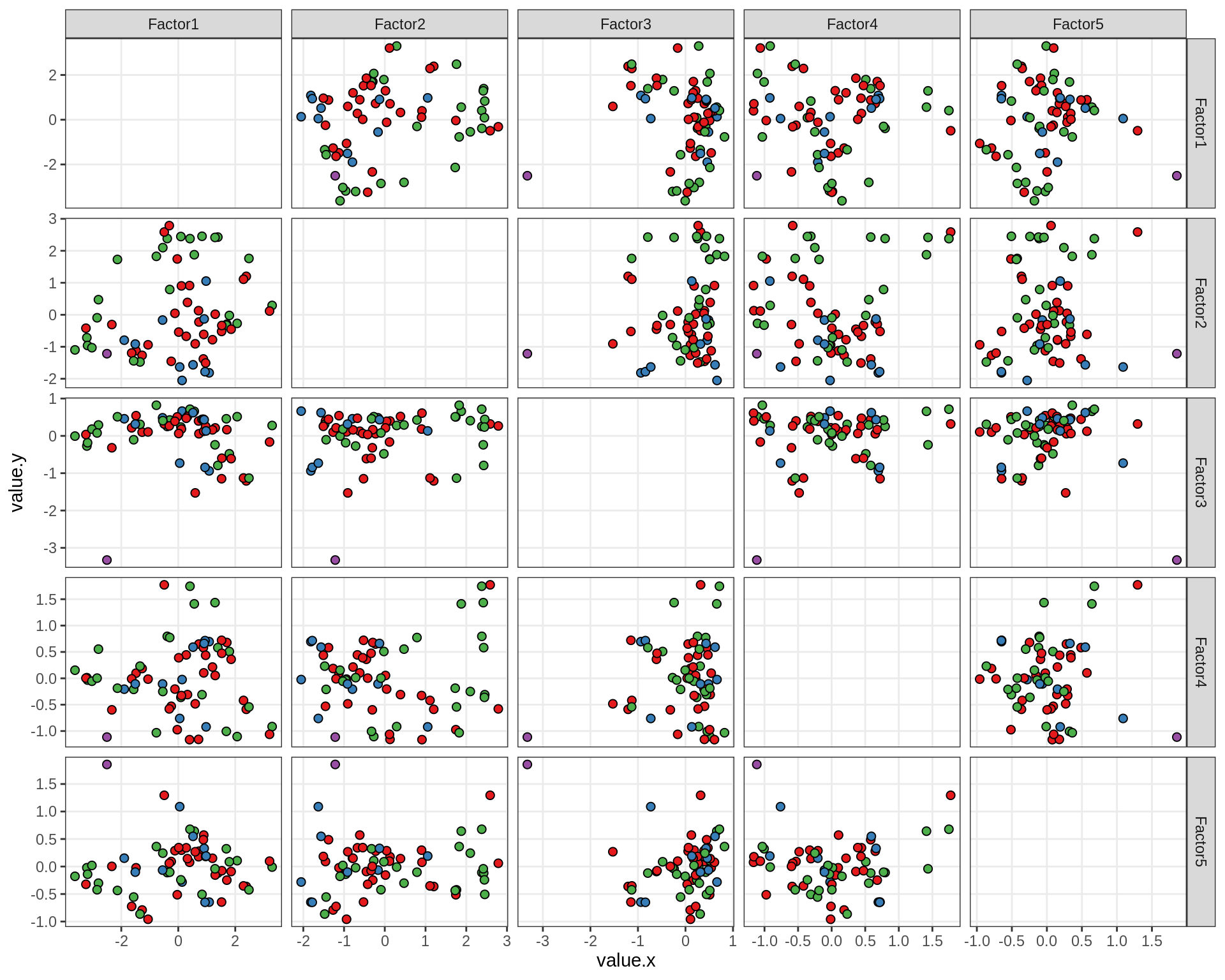
}Factor distributions - pairwise
for (annot in c('subject_id', 'lesion_type', 'diagnosis', 'sex', 'sample_source', 'smoker', 'comp_grp')) {
cat('### by ', annot, '\n', sep = '')
print(plot_factors_pairwise(model, annots_dt, pb, by = annot))
cat('\n\n')
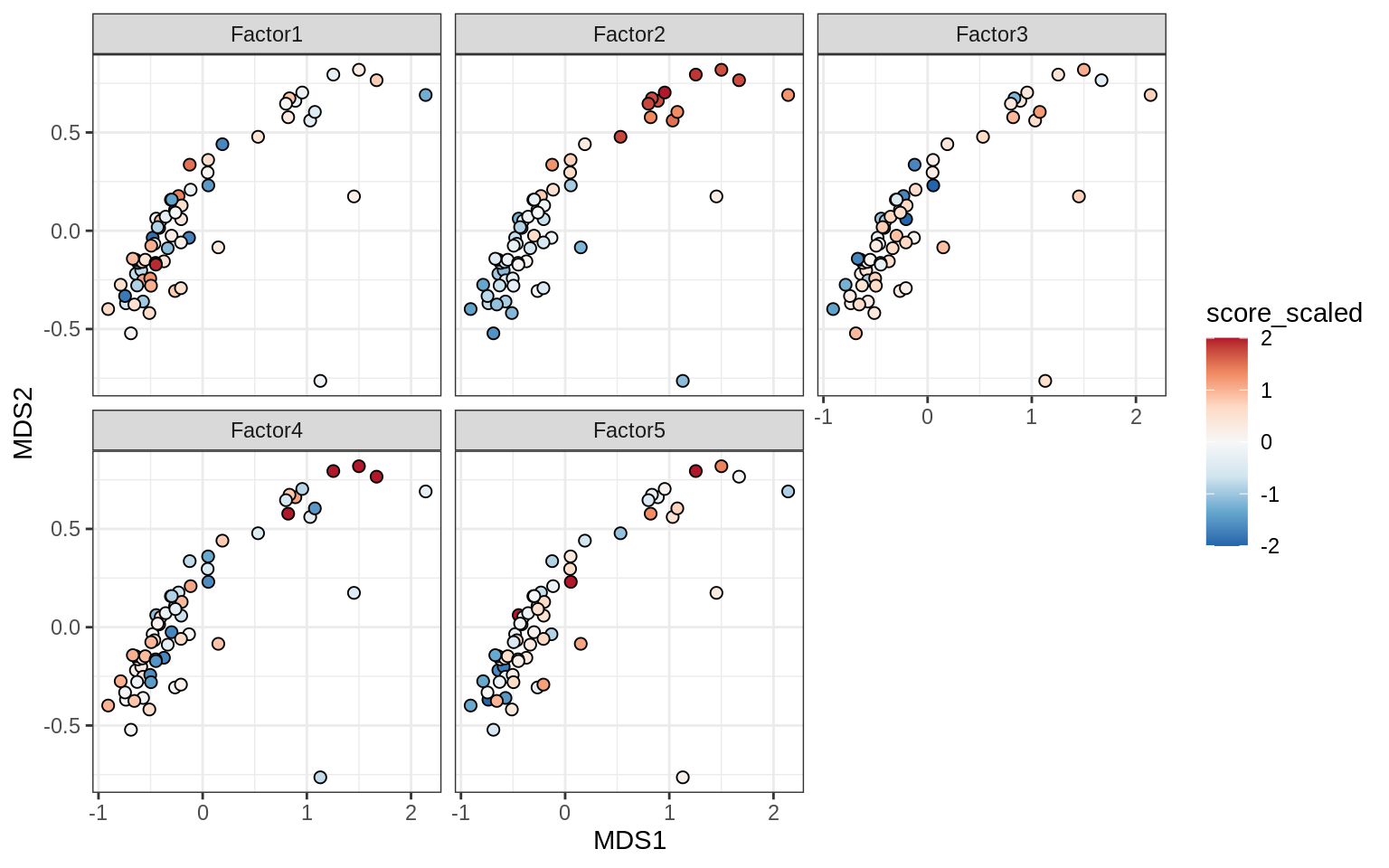
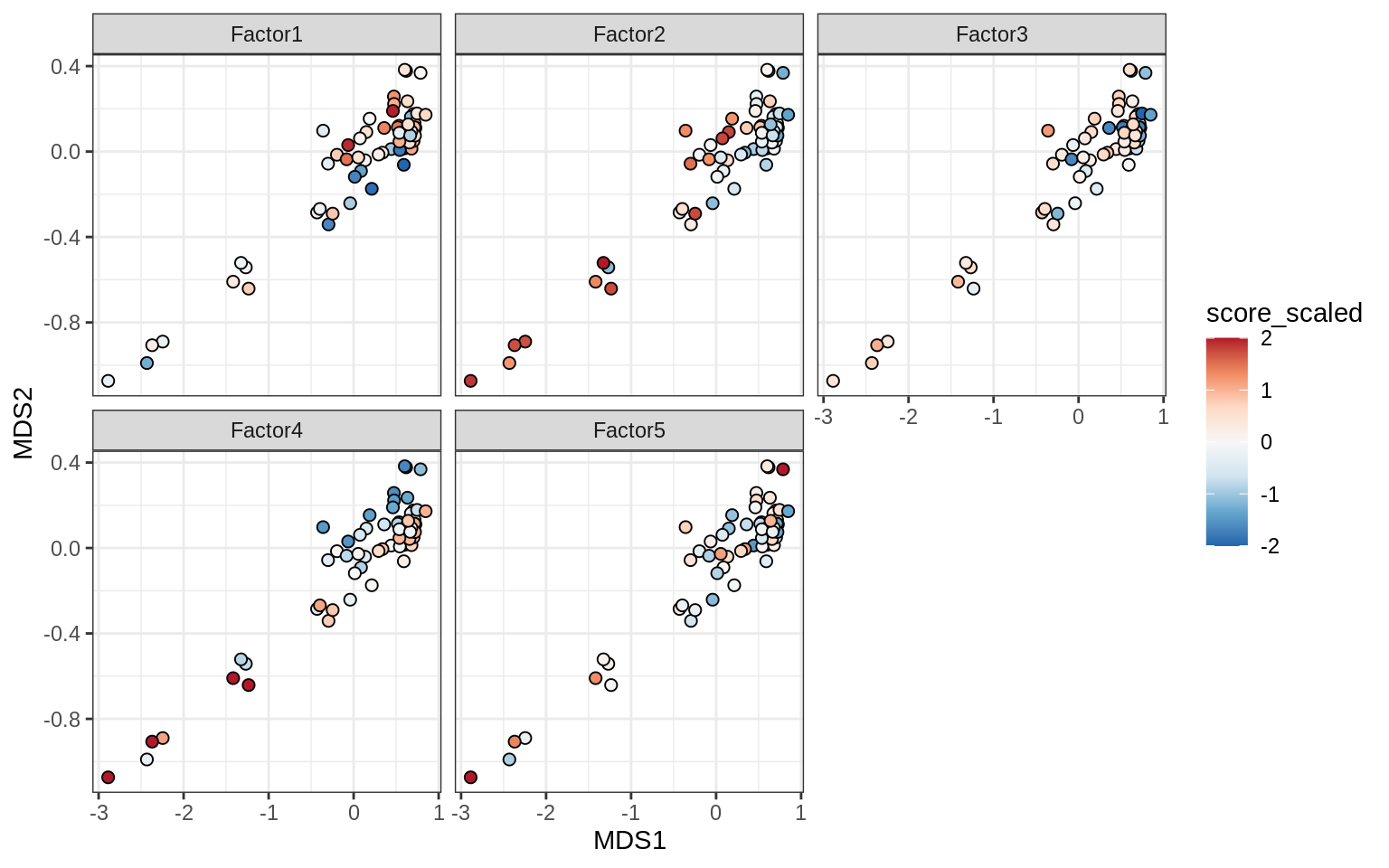
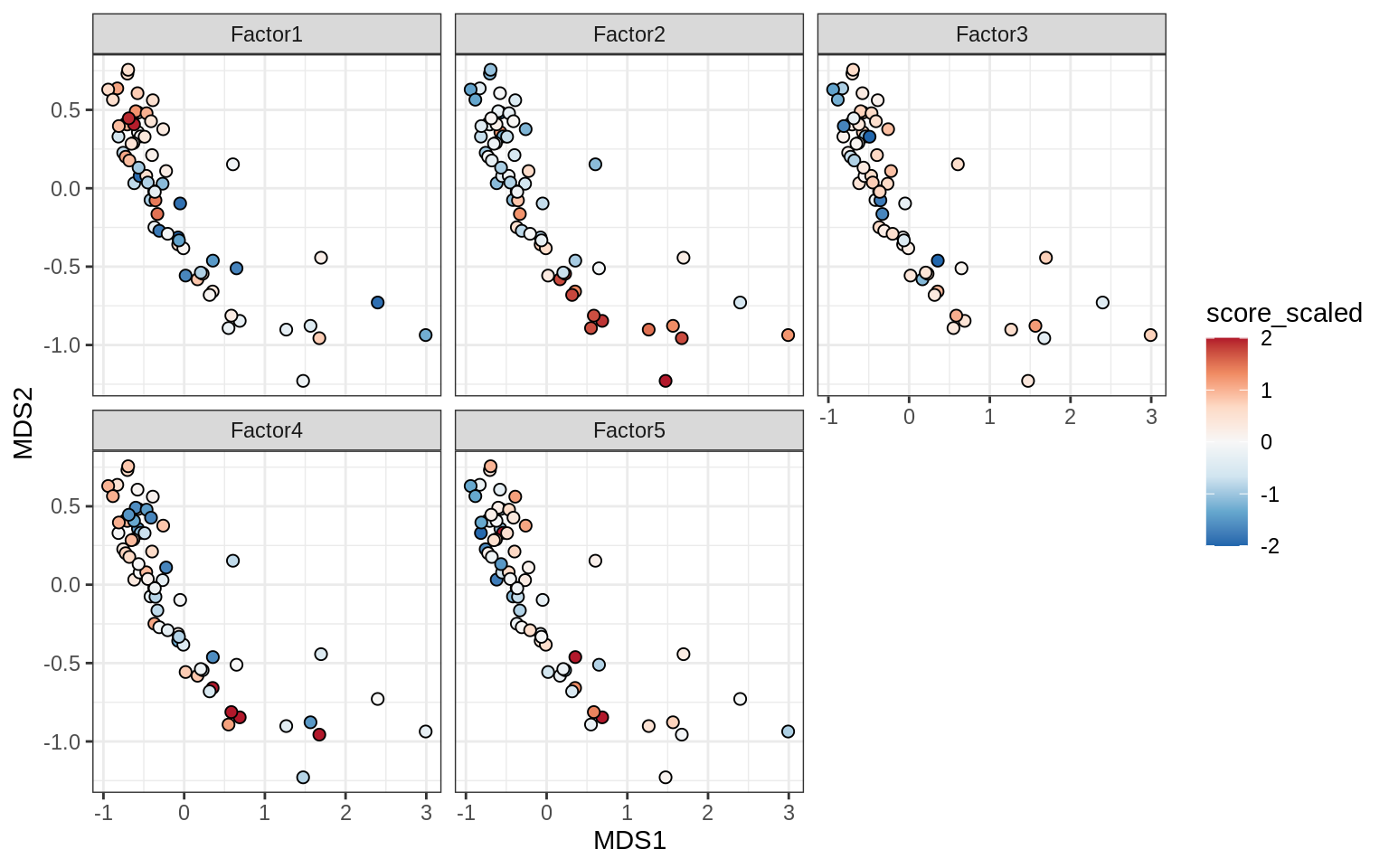
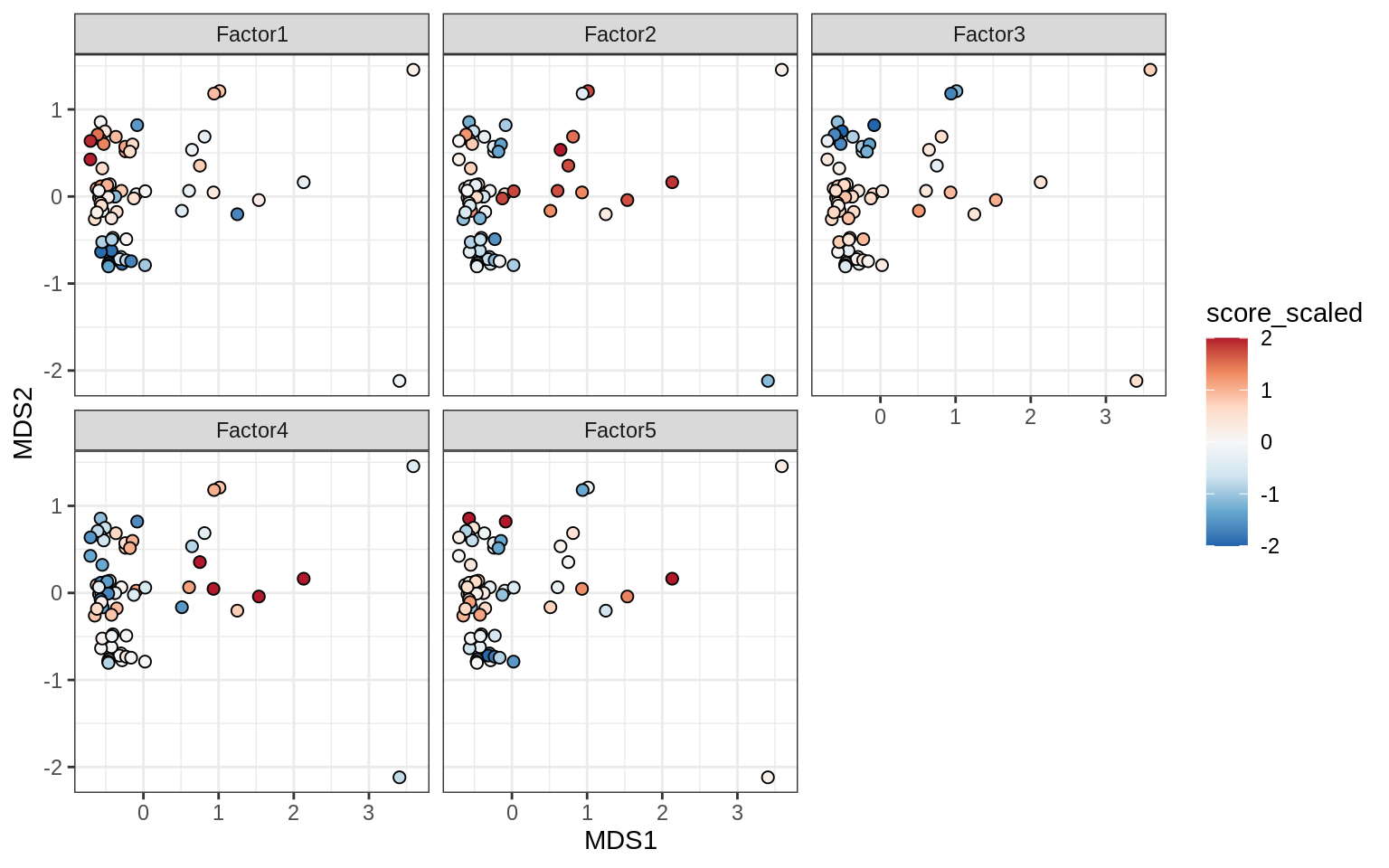
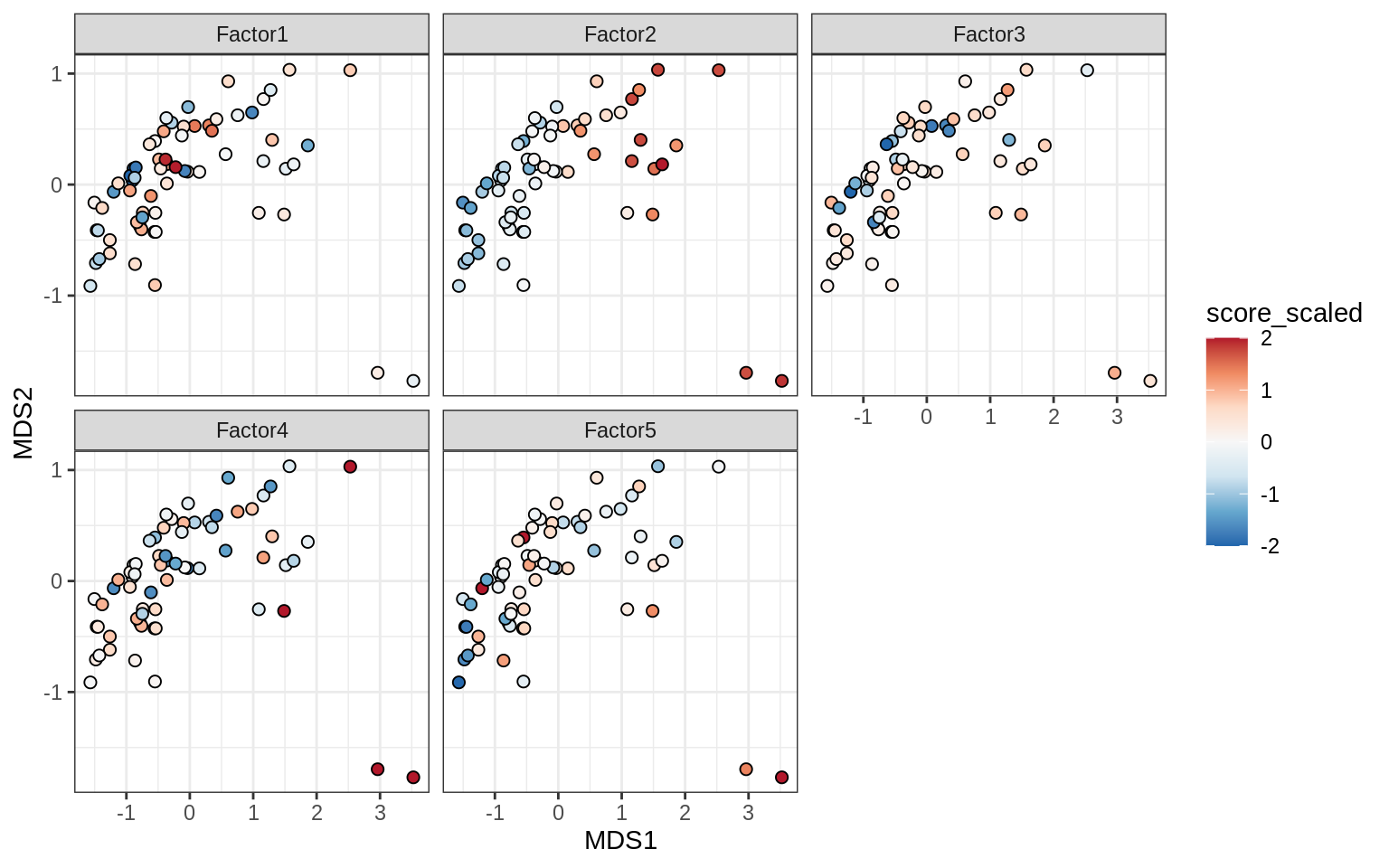
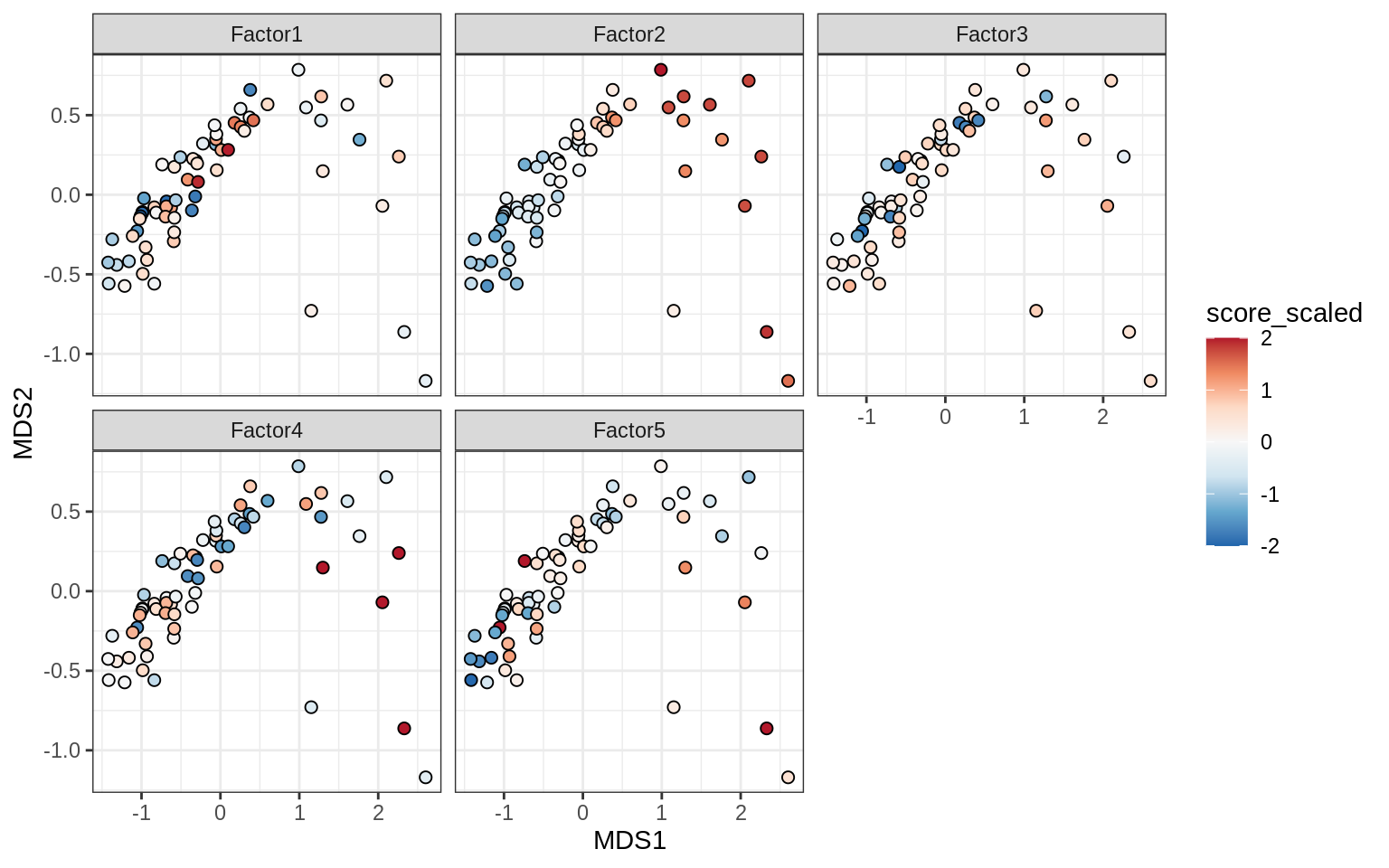
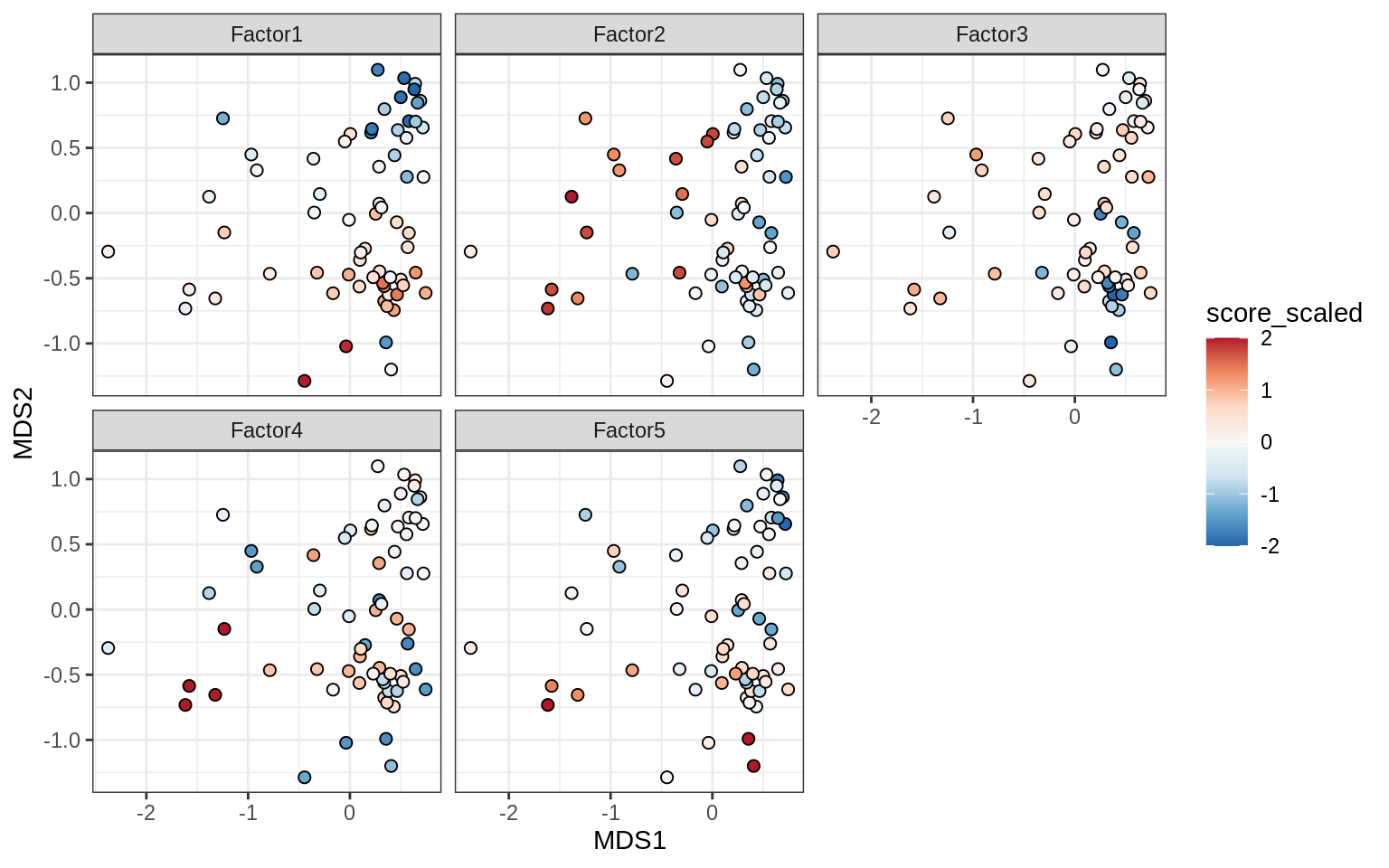
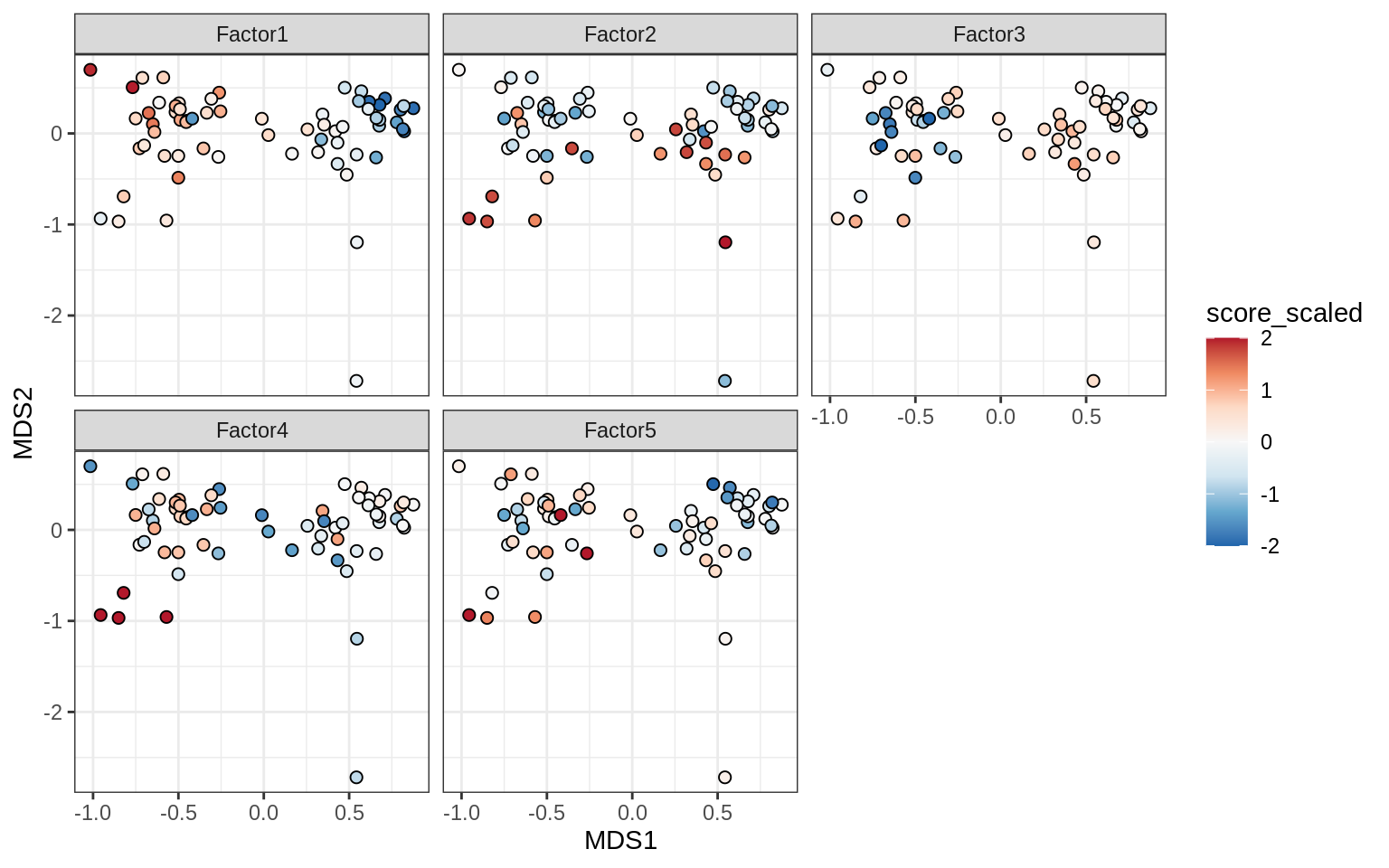
}Factors over MDS layouts
for (cl in broad_ord) {
if (!(broad_short[[cl]] %in% views_names(model)))
next
cat('### ', cl, '\n', sep = '')
print(plot_factors_over_mds_samples(model, mds_sep_dt, cl = cl))
cat('\n\n')
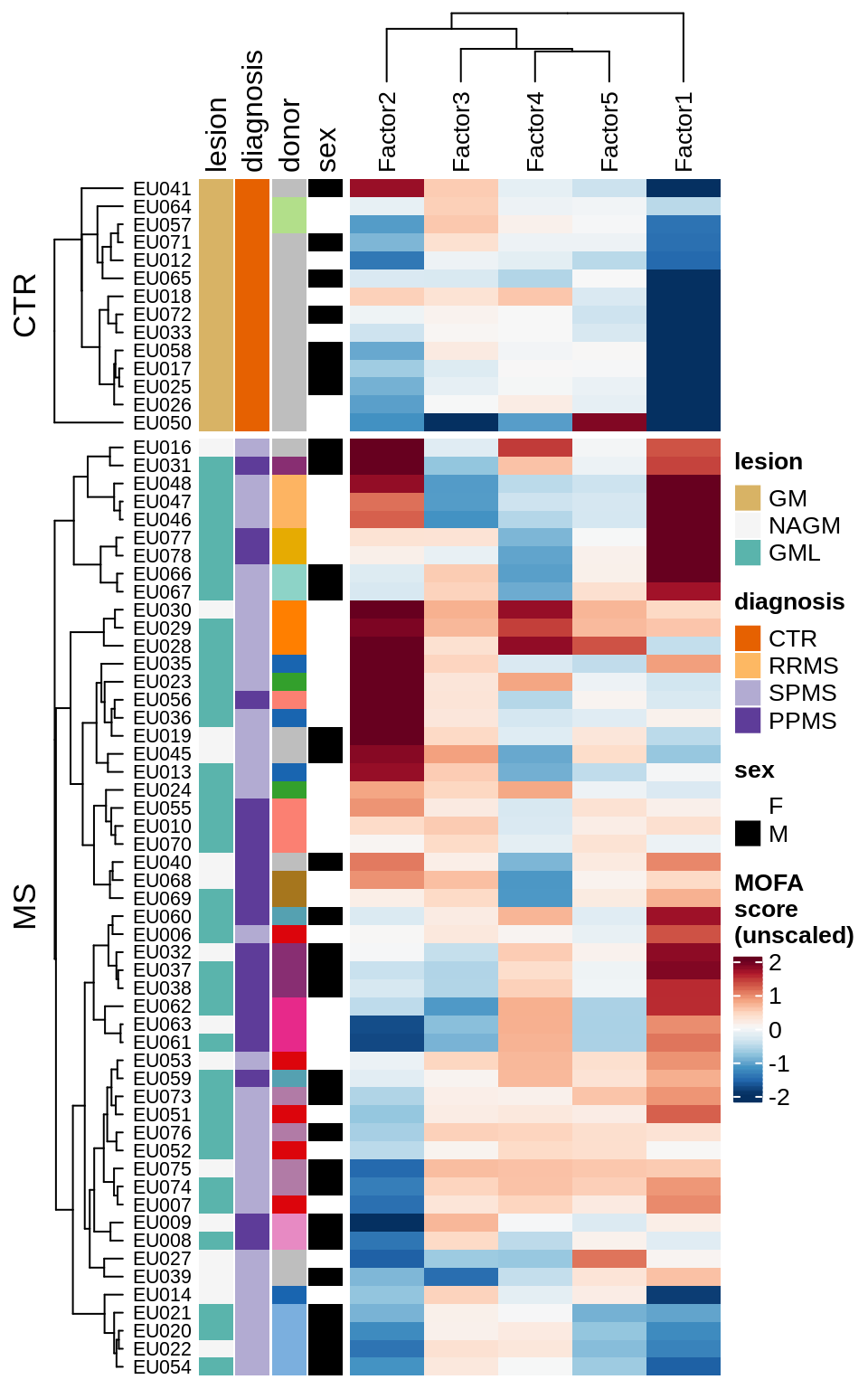
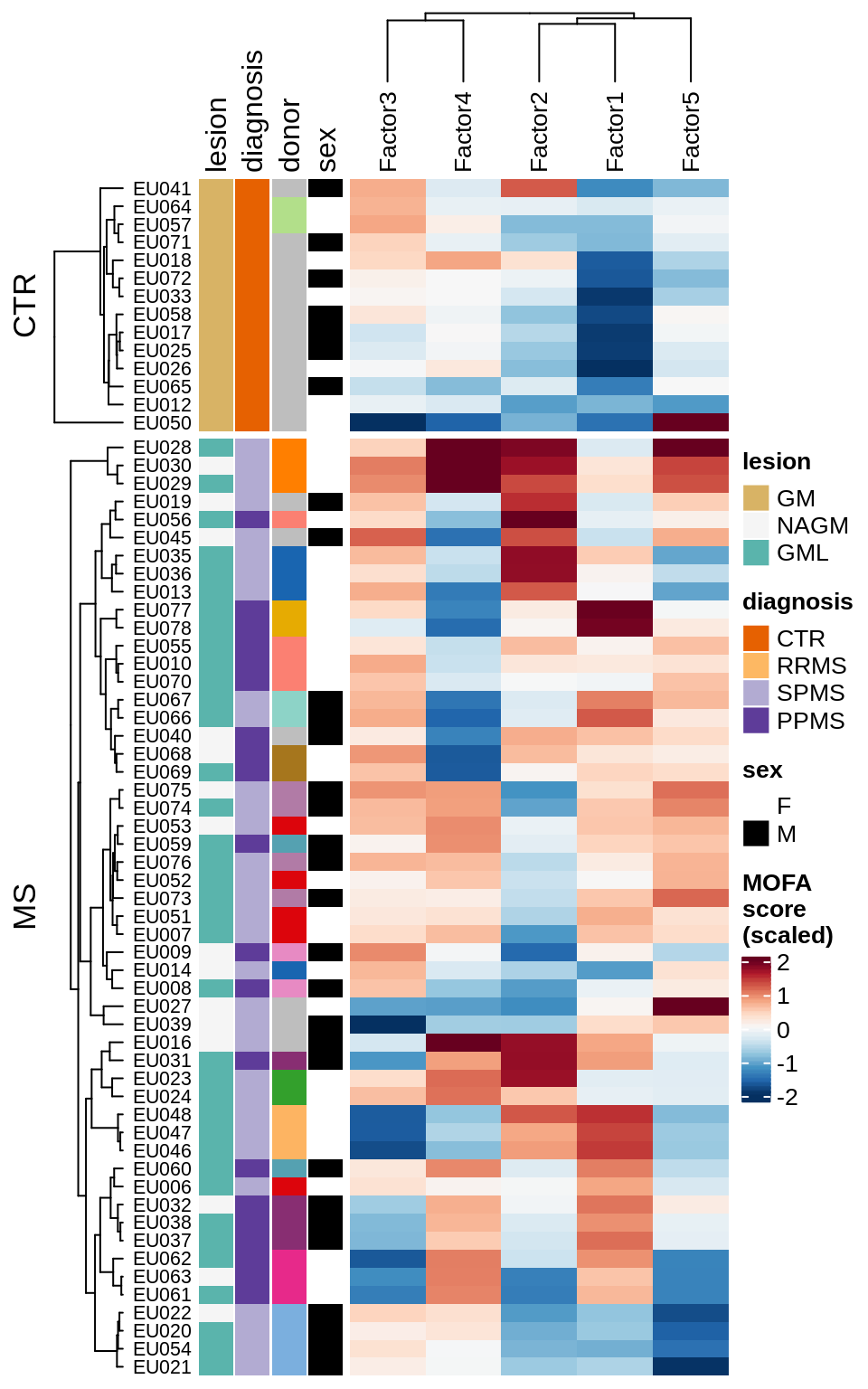
}Factor distributions with patient annotations - few
for (v in c('score', 'score_scaled')) {
cat('### ', v, '\n', sep = '')
draw(plot_factors_heatmap(model, annots_dt, pb, what = 'few', plot_var = v))
cat('\n\n')
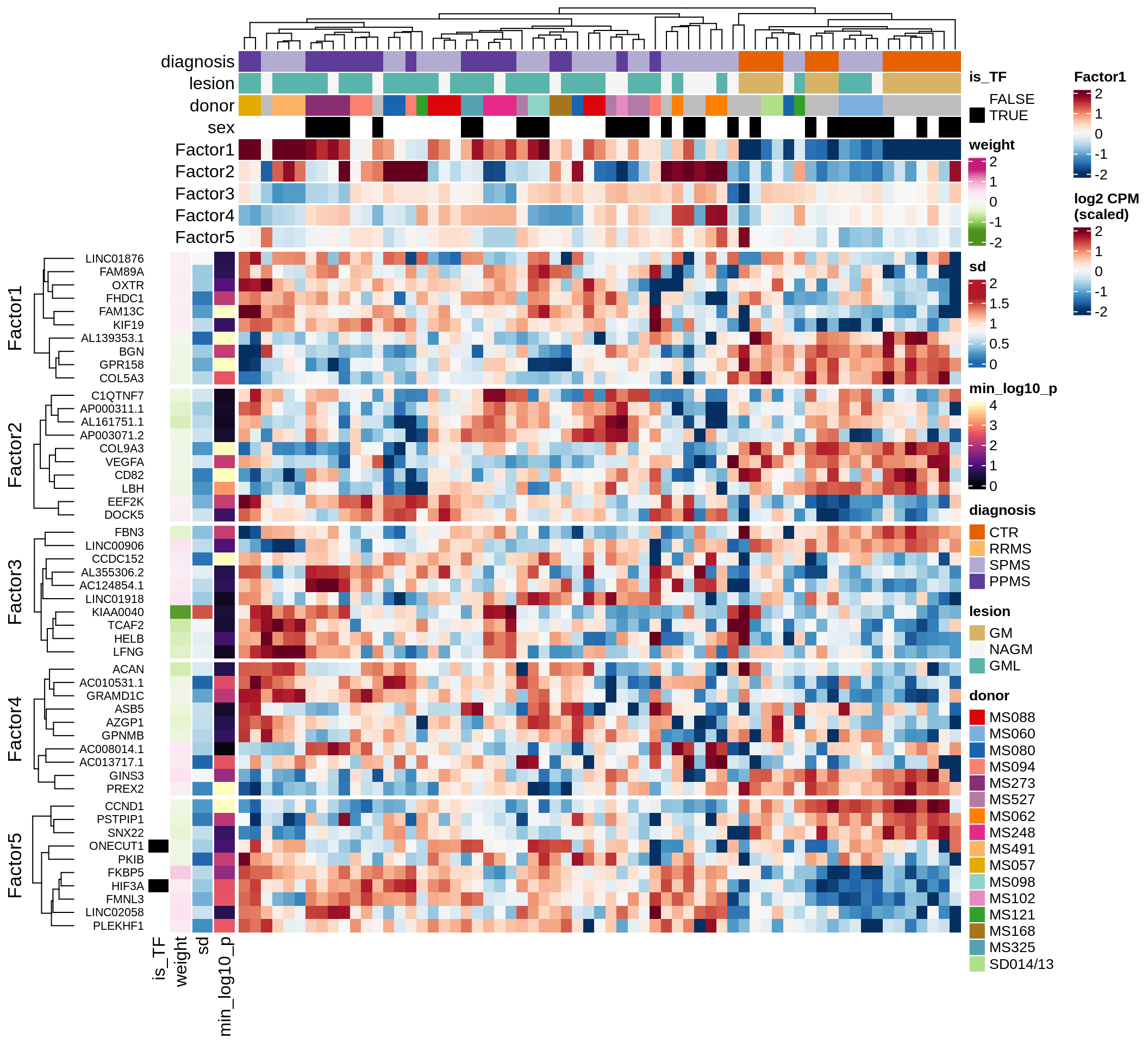
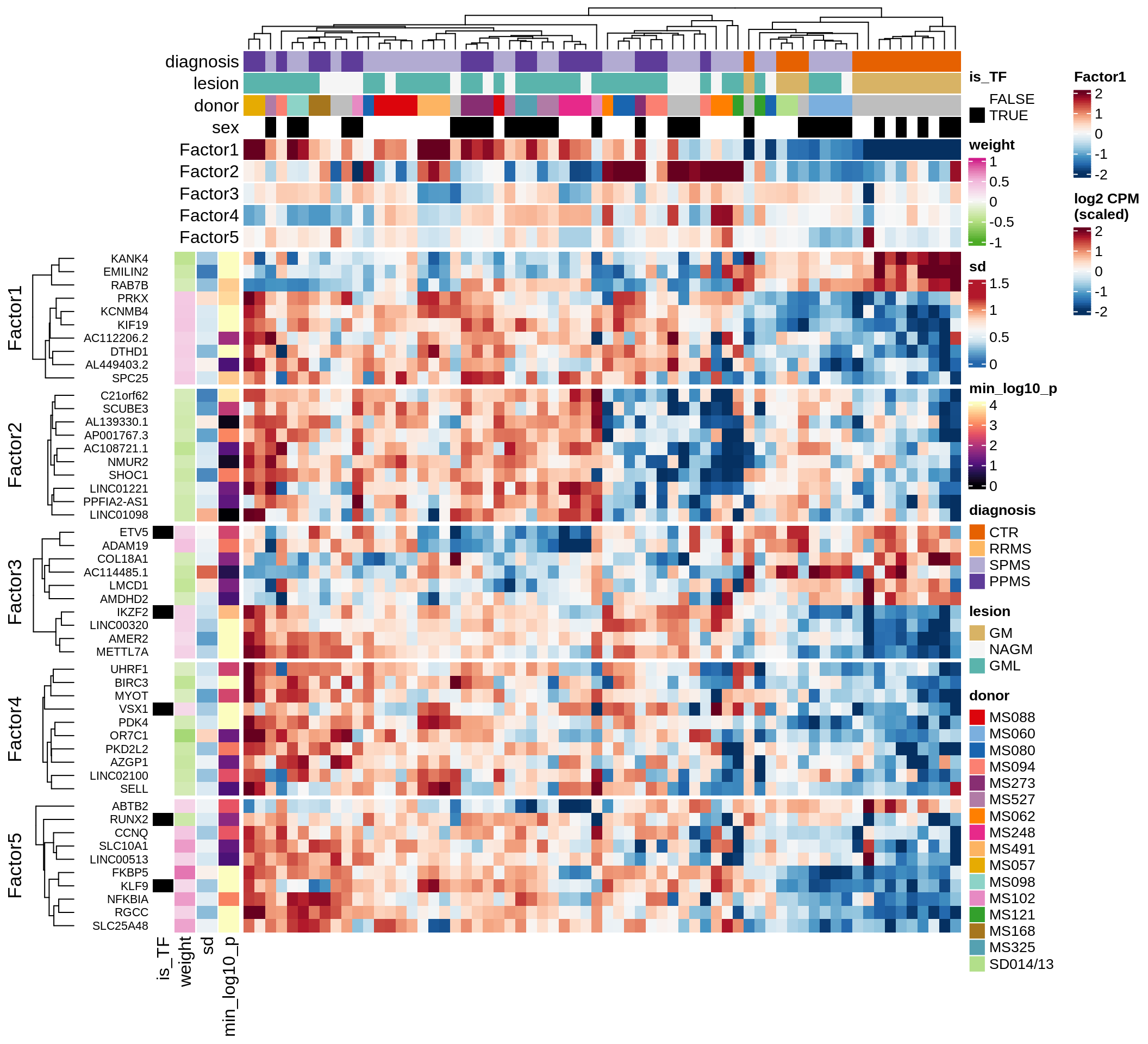
}Factor distributions with patient annotations - all
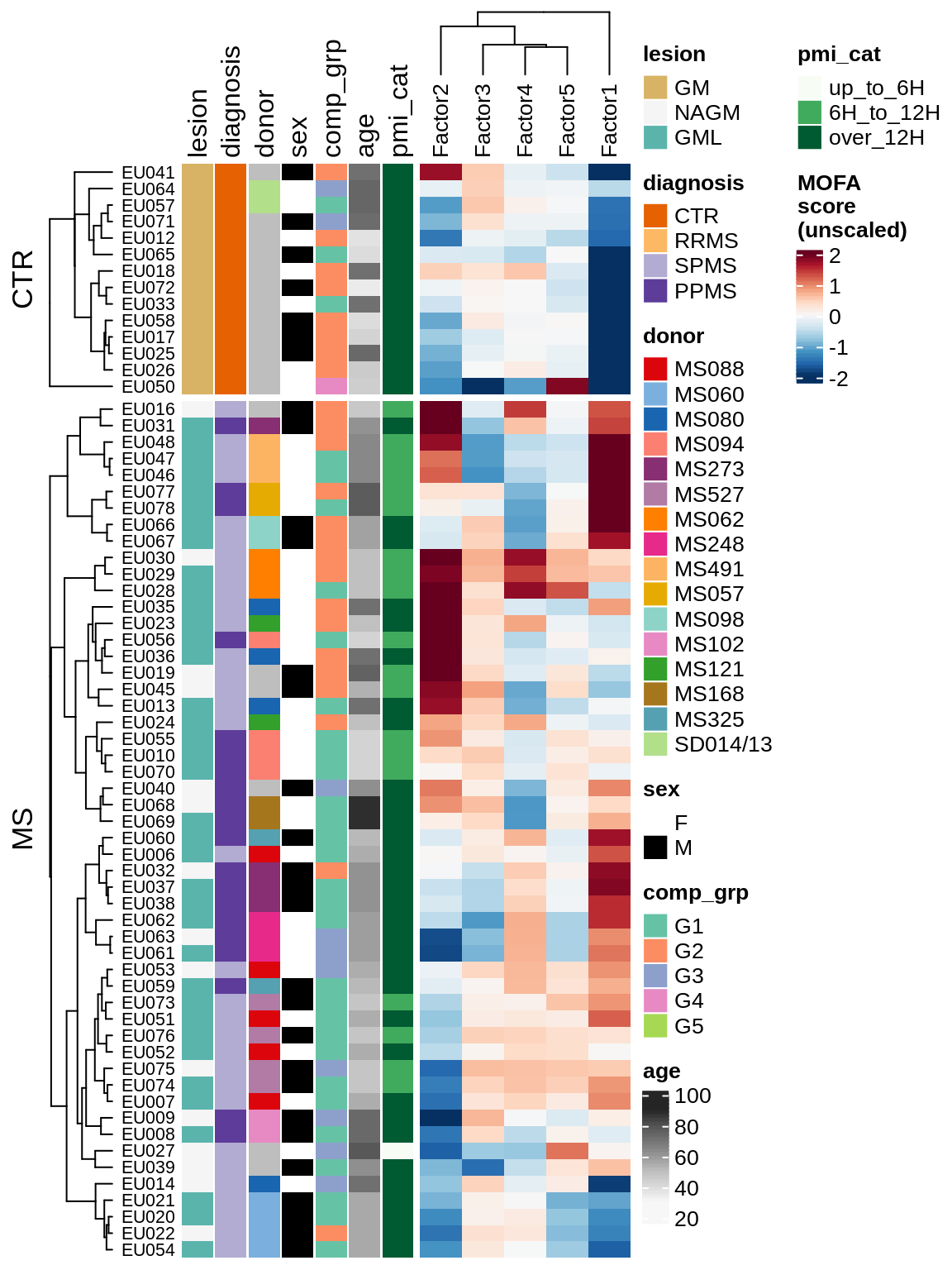
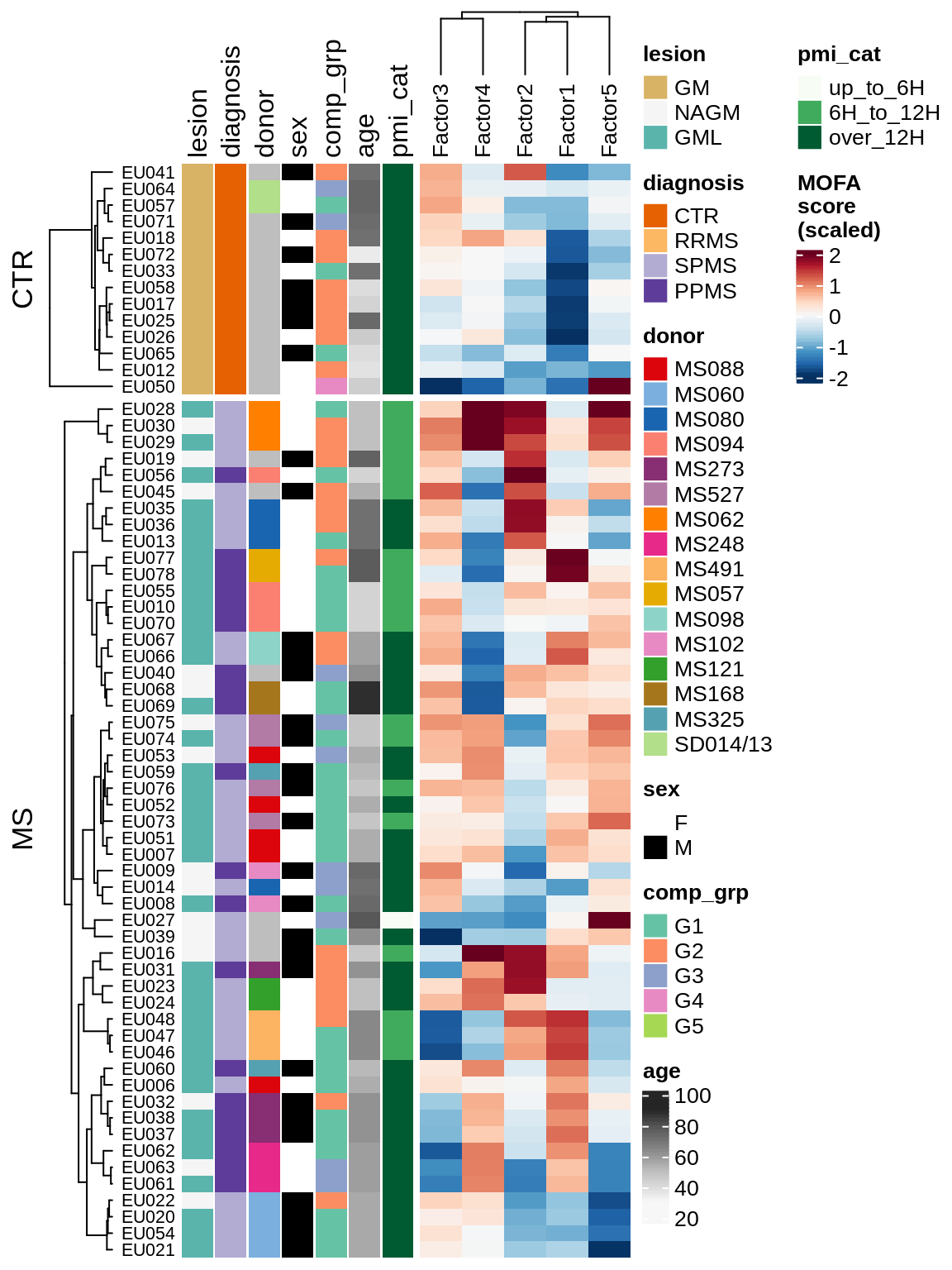
for (v in c('score', 'score_scaled')) {
cat('### ', v, '\n', sep = '')
draw(plot_factors_heatmap(model, annots_dt, pb, what = 'all', plot_var = v))
cat('\n\n')
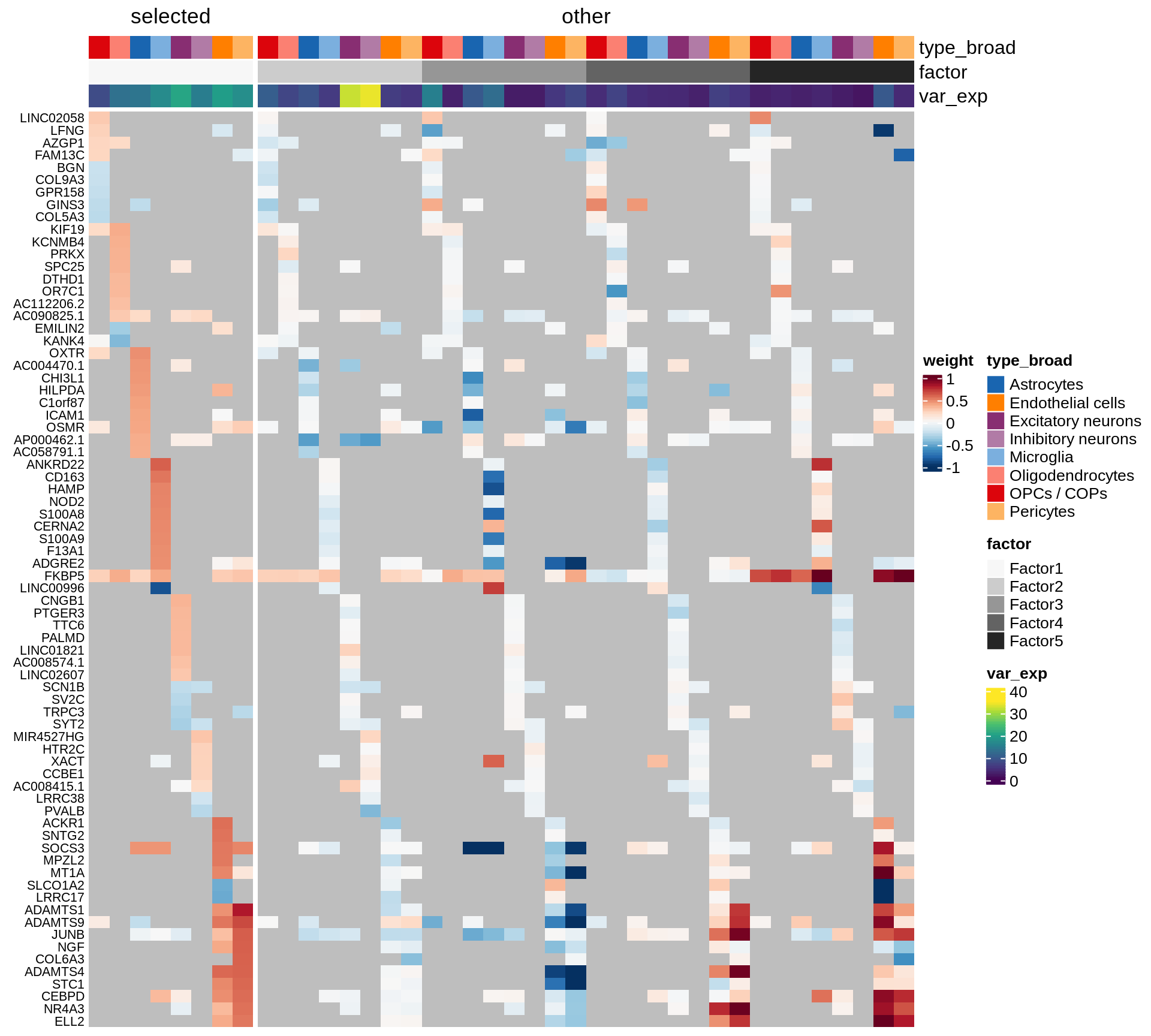
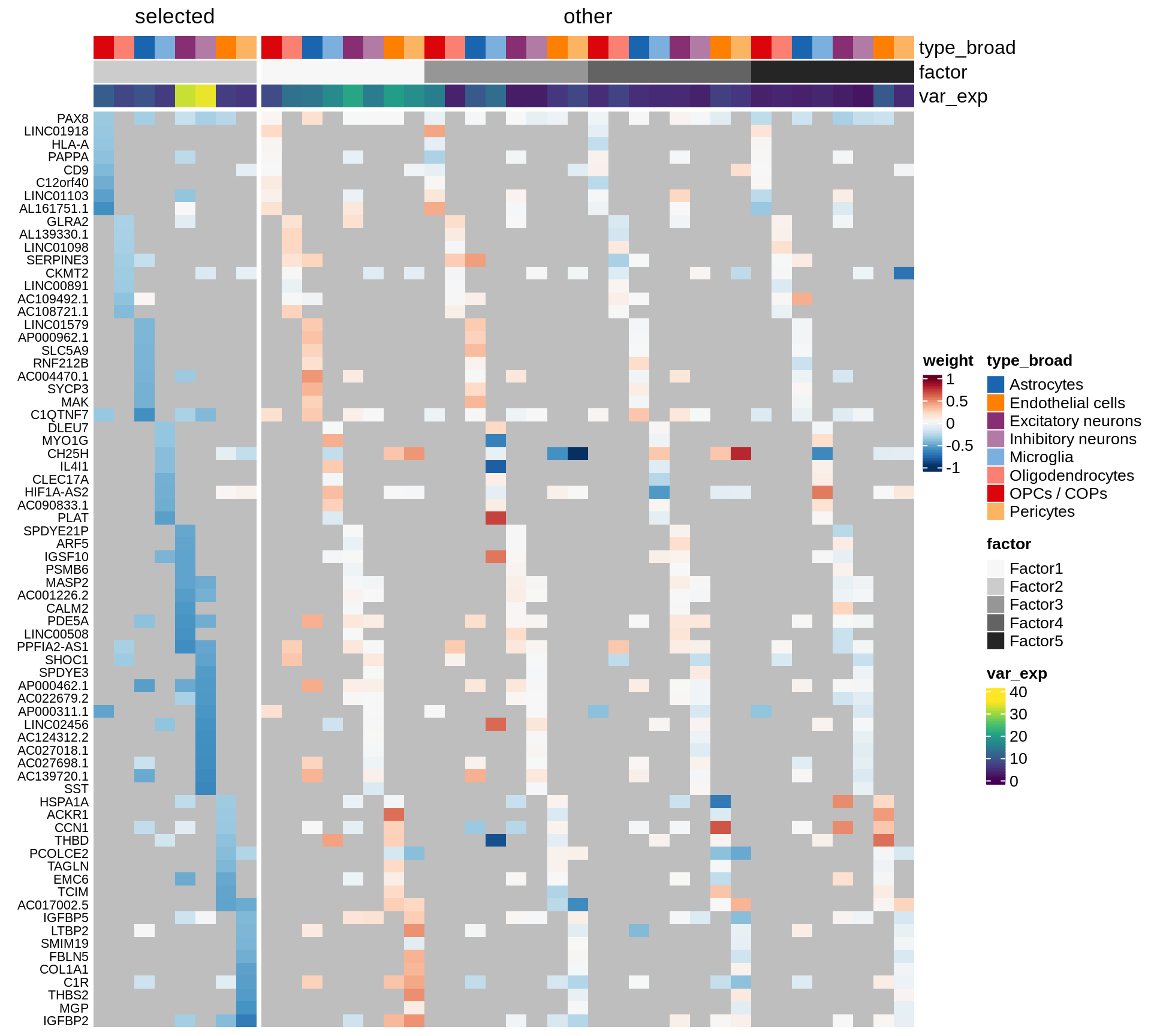
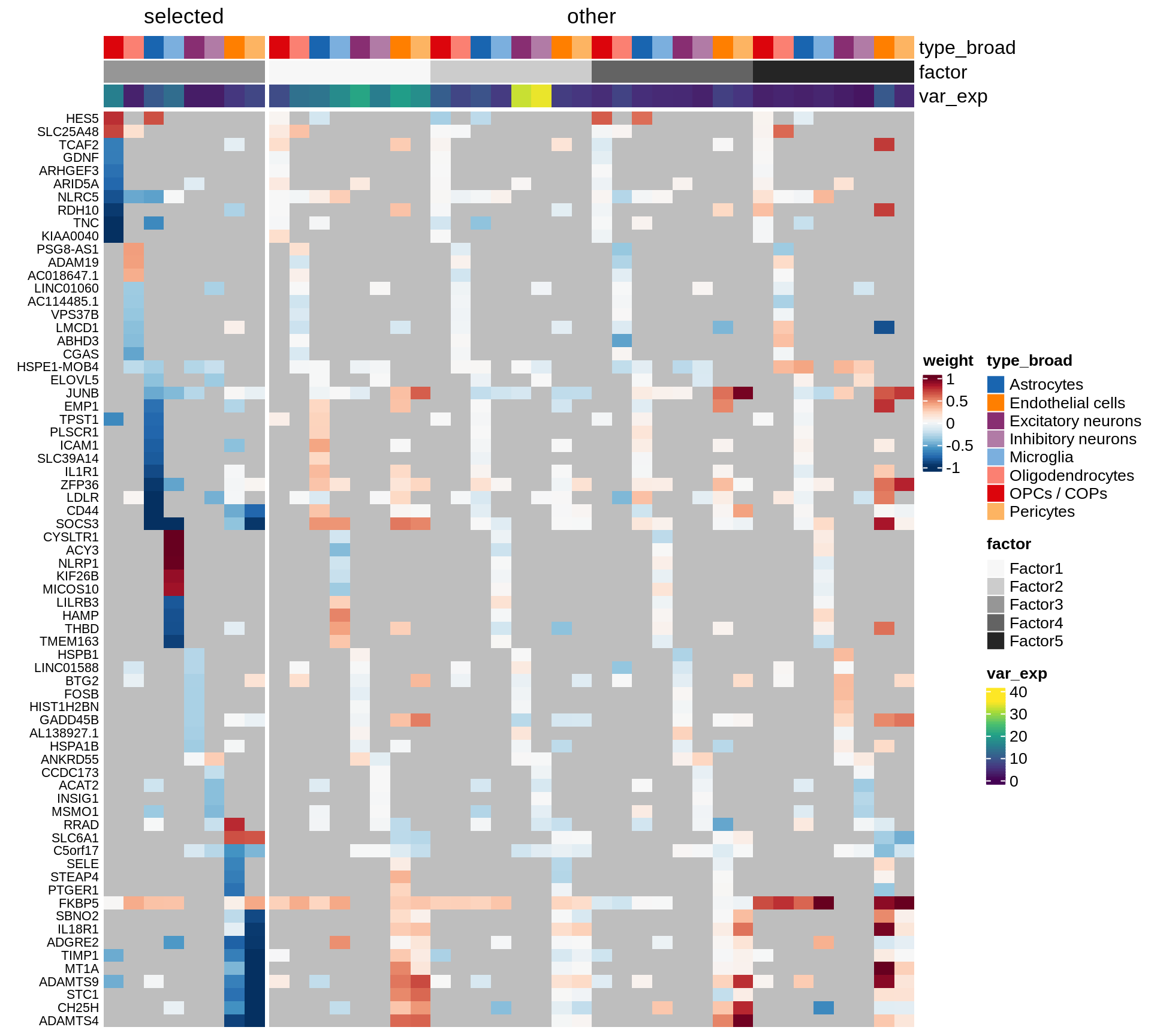
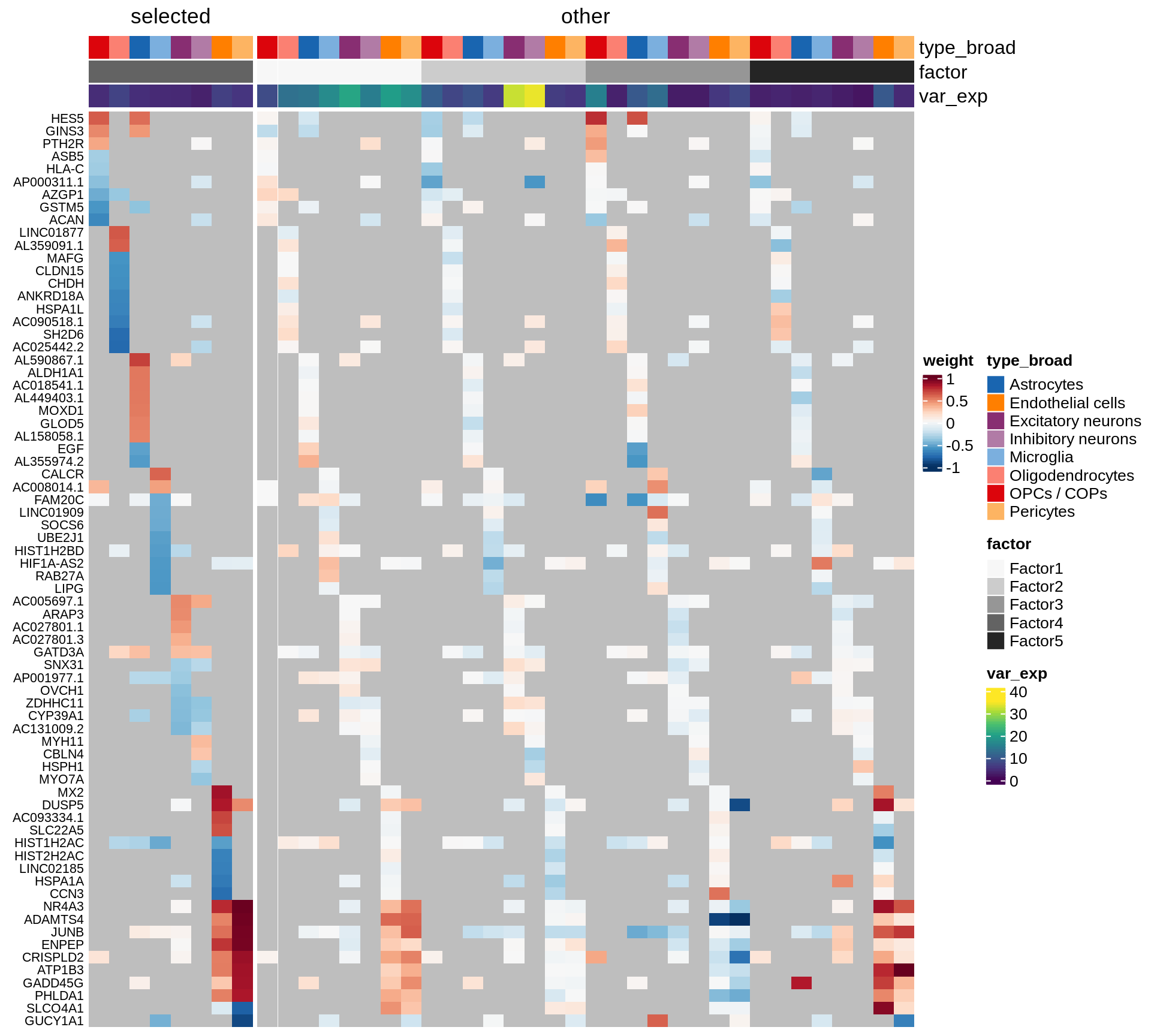
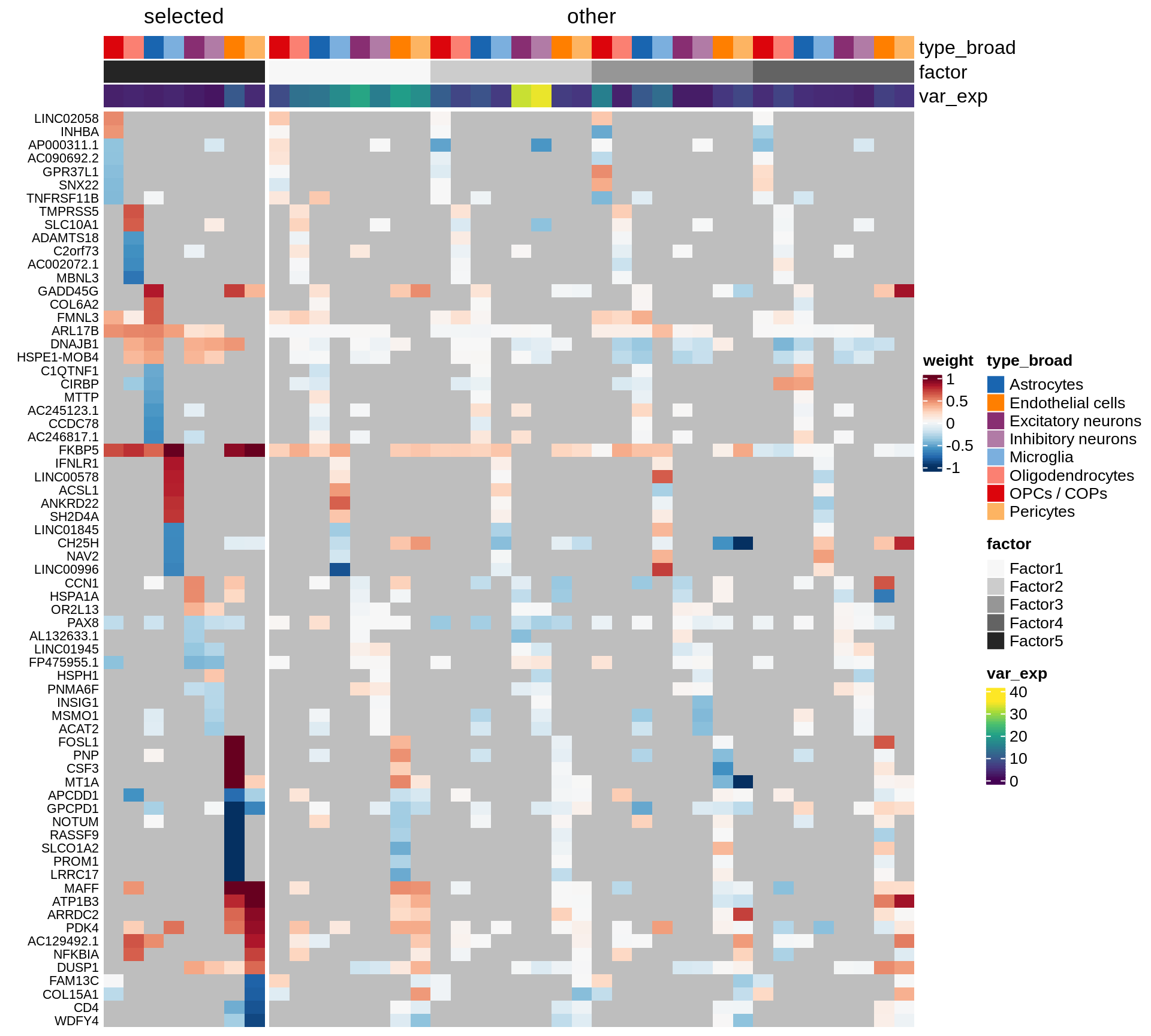
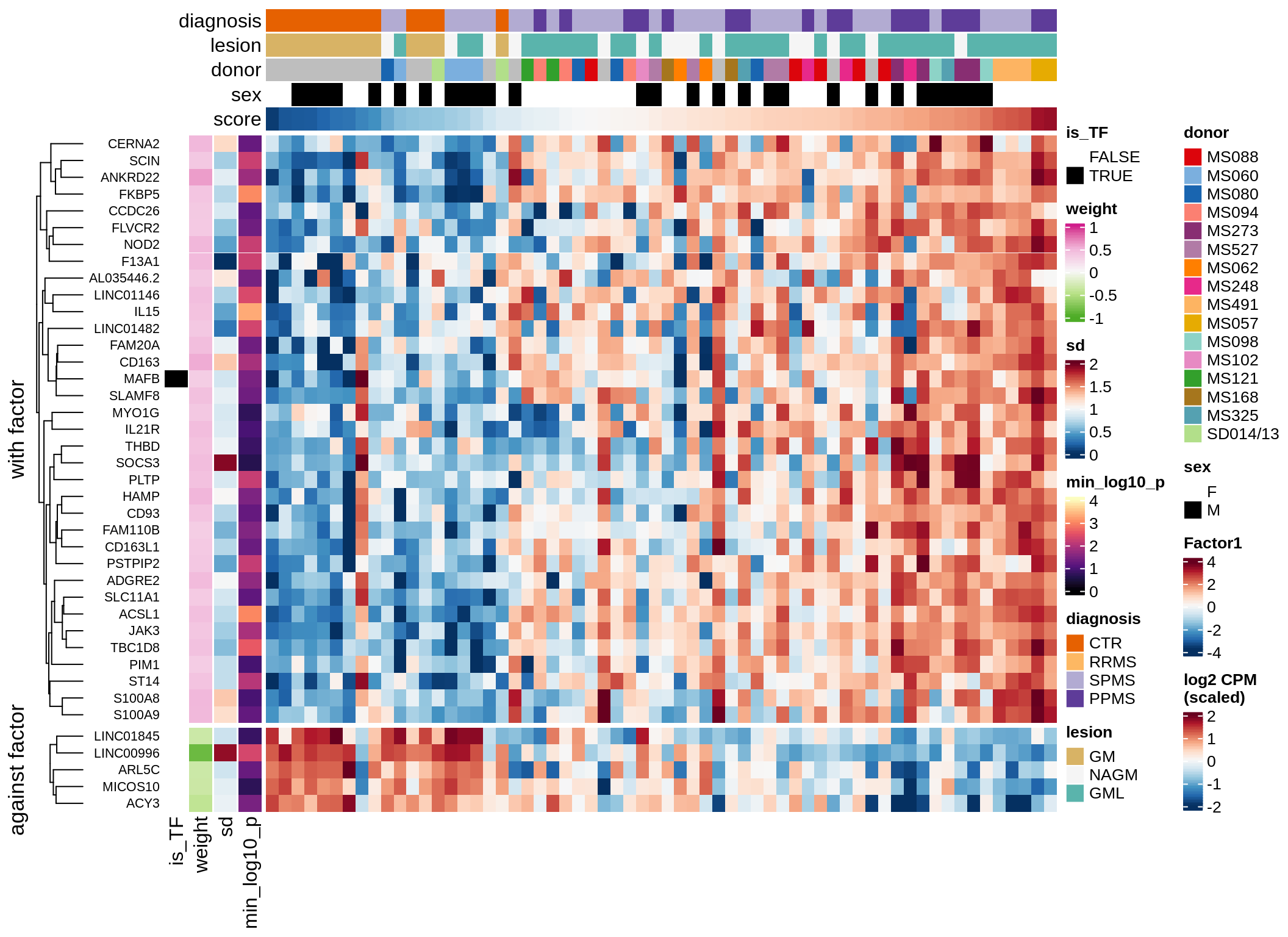
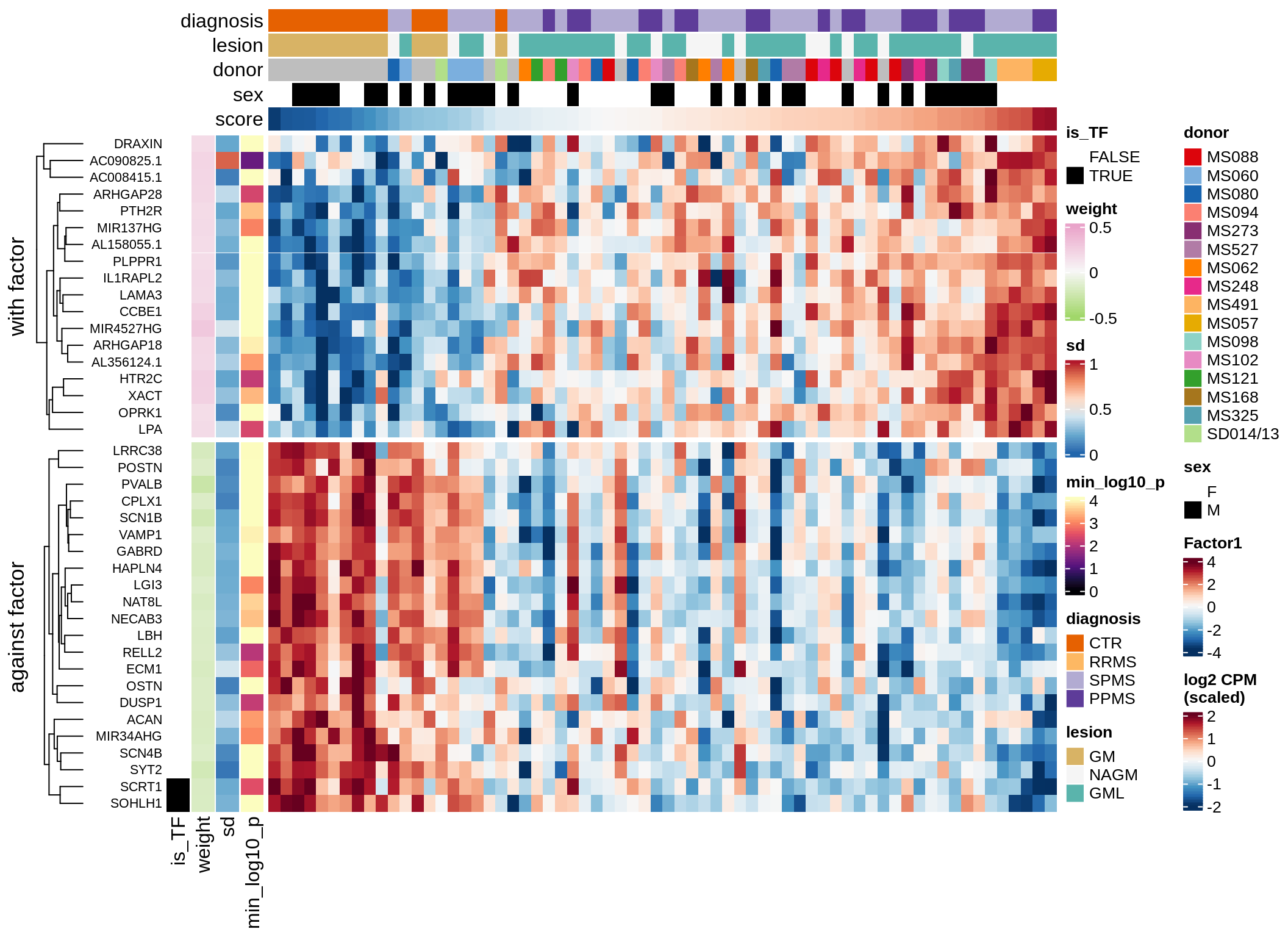
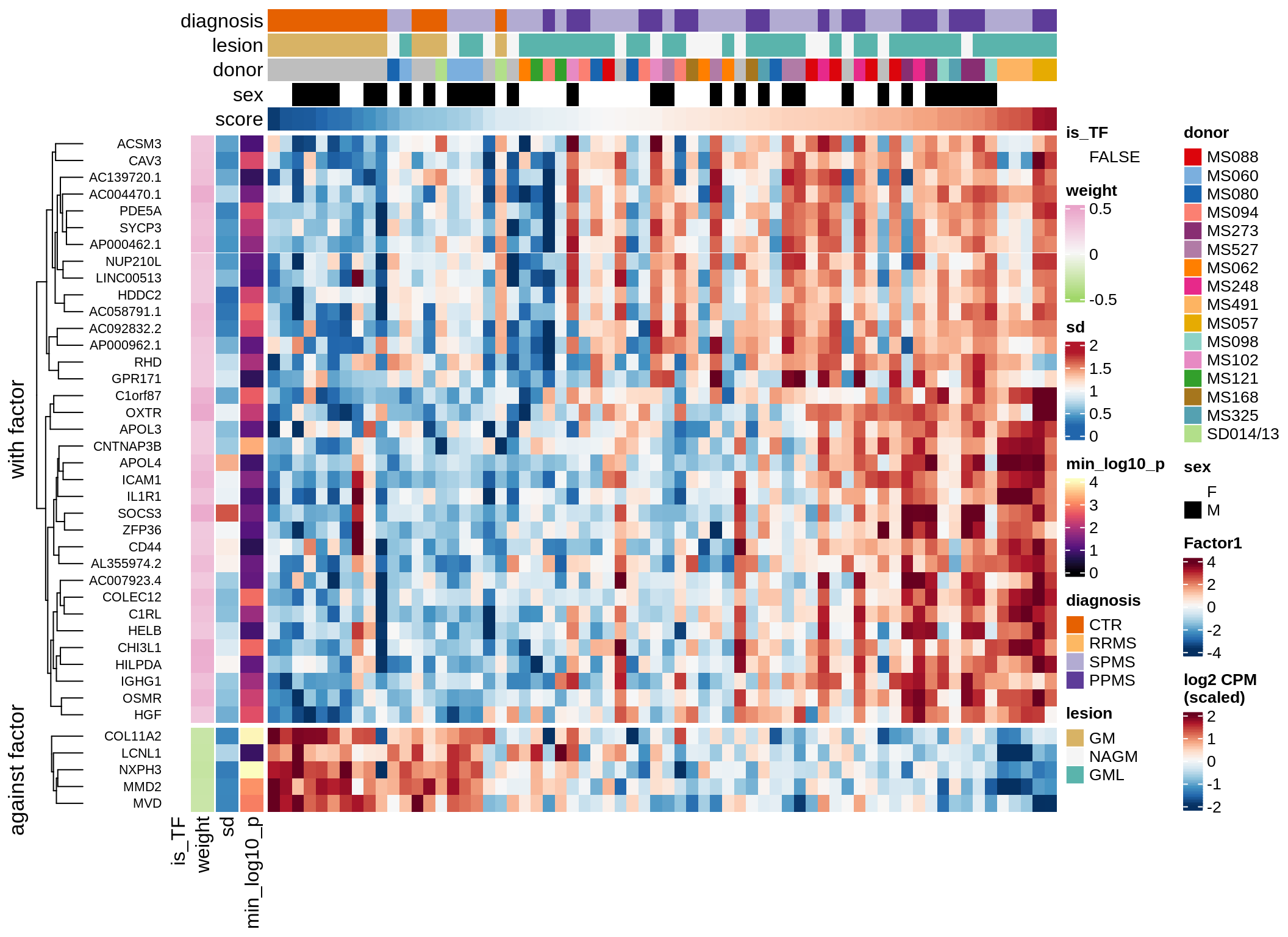
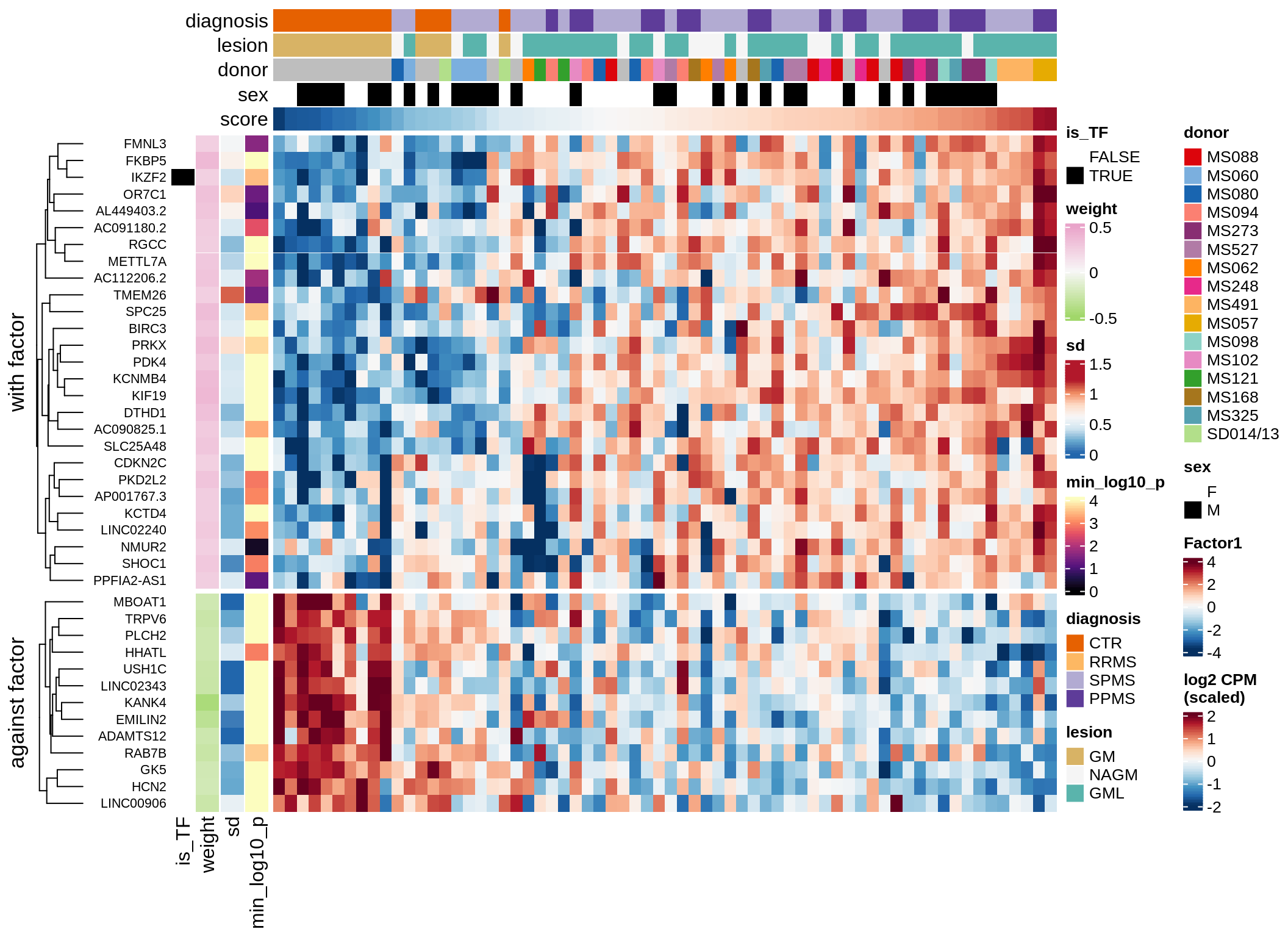
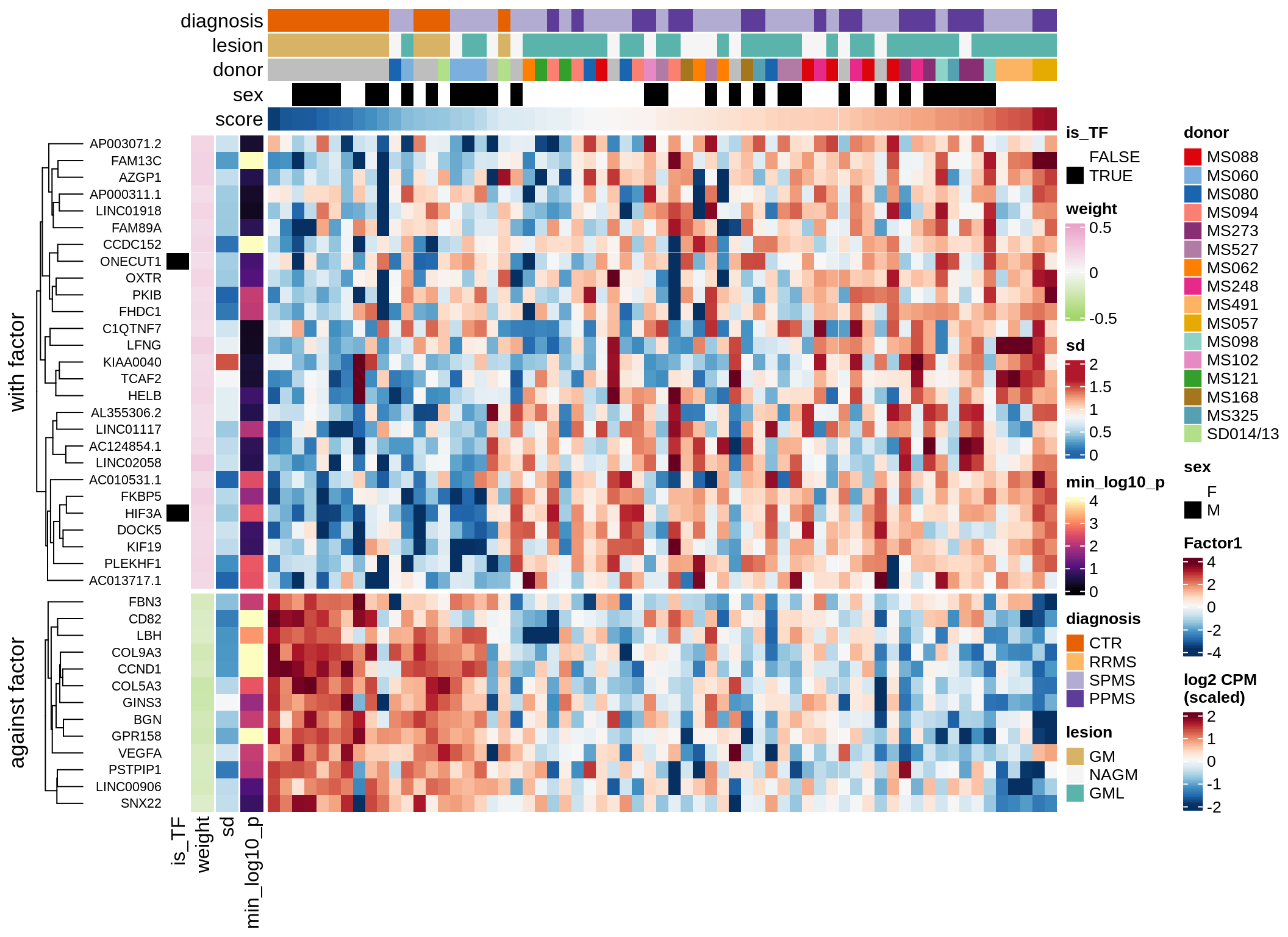
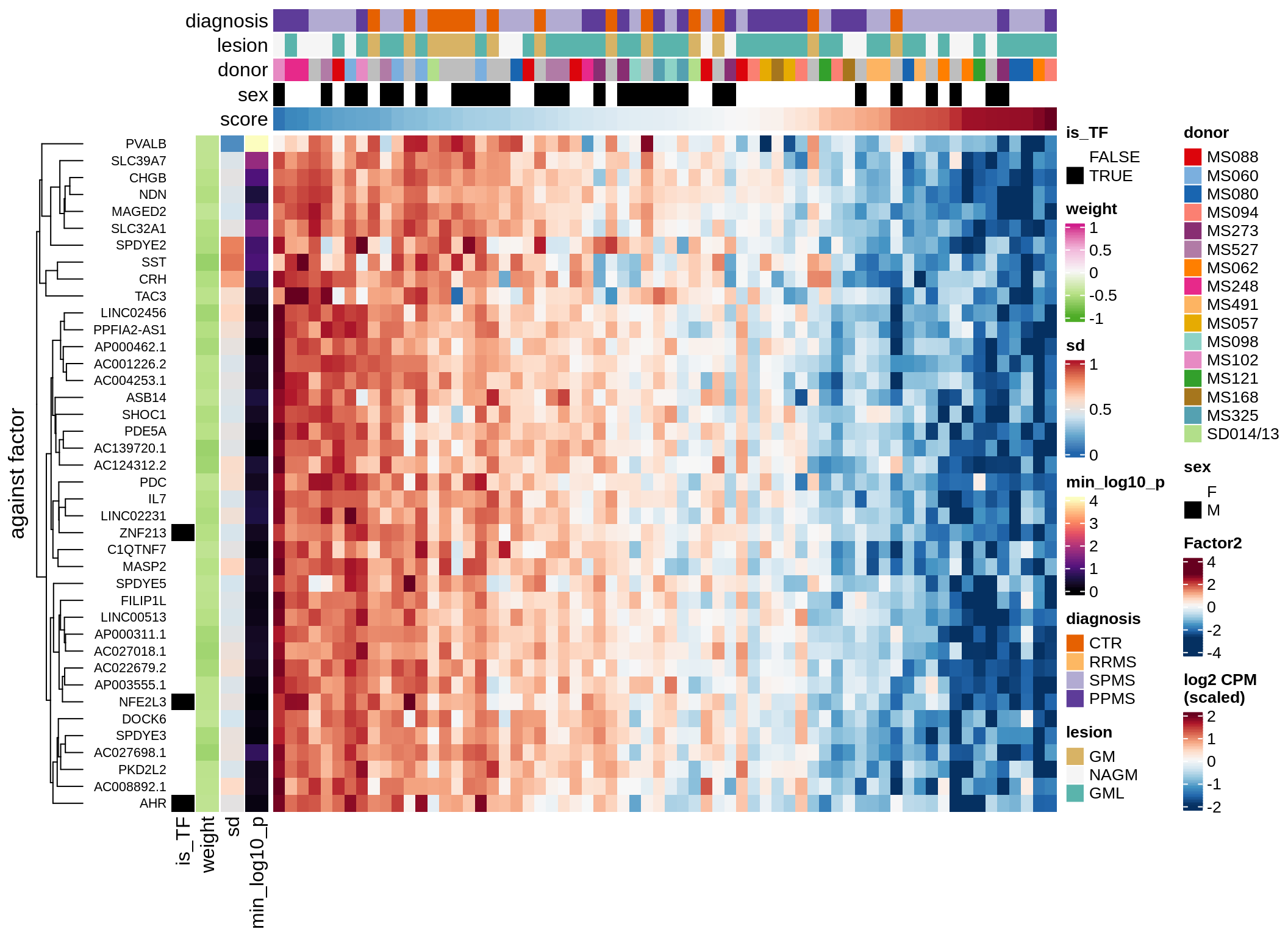
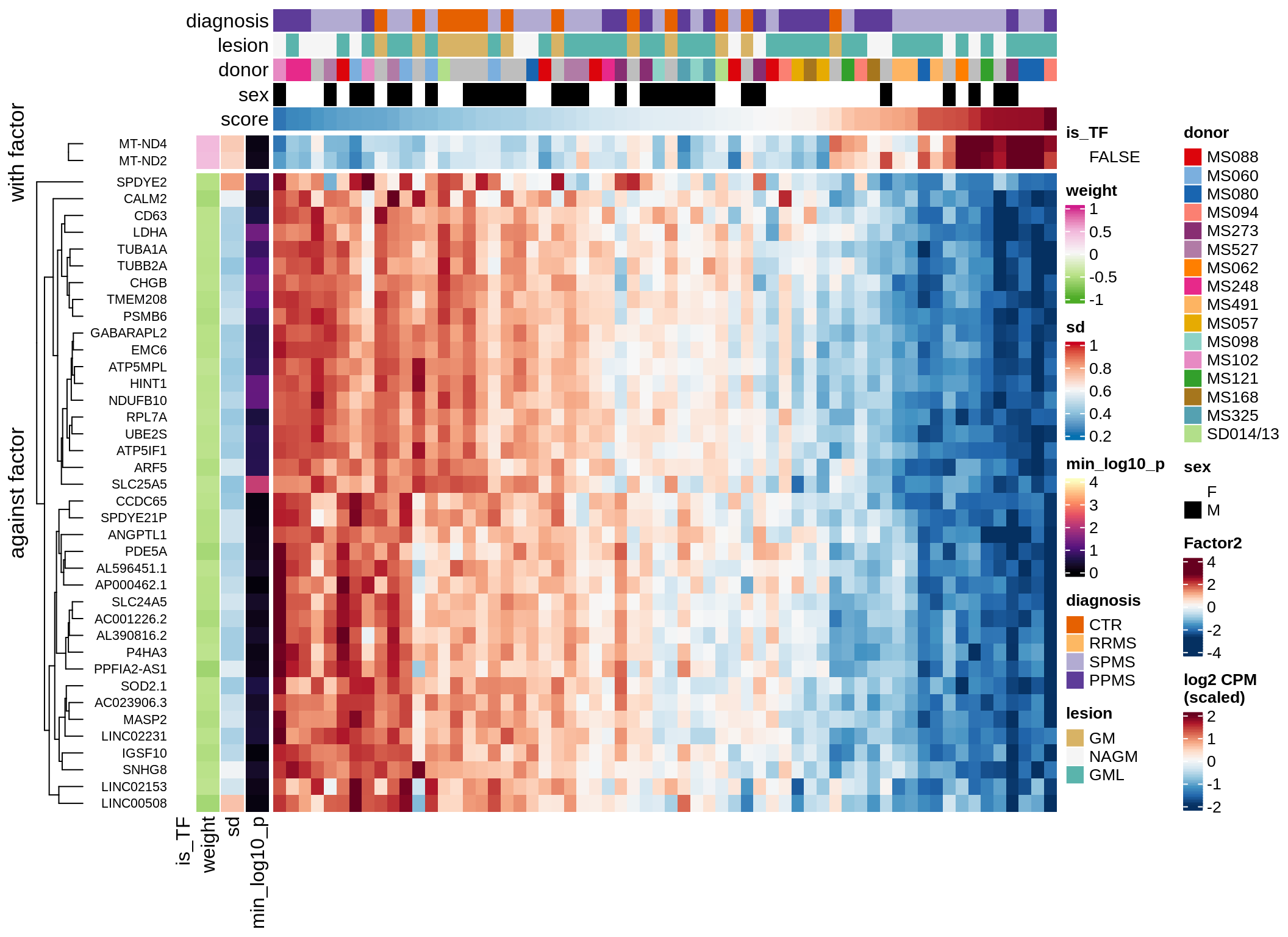
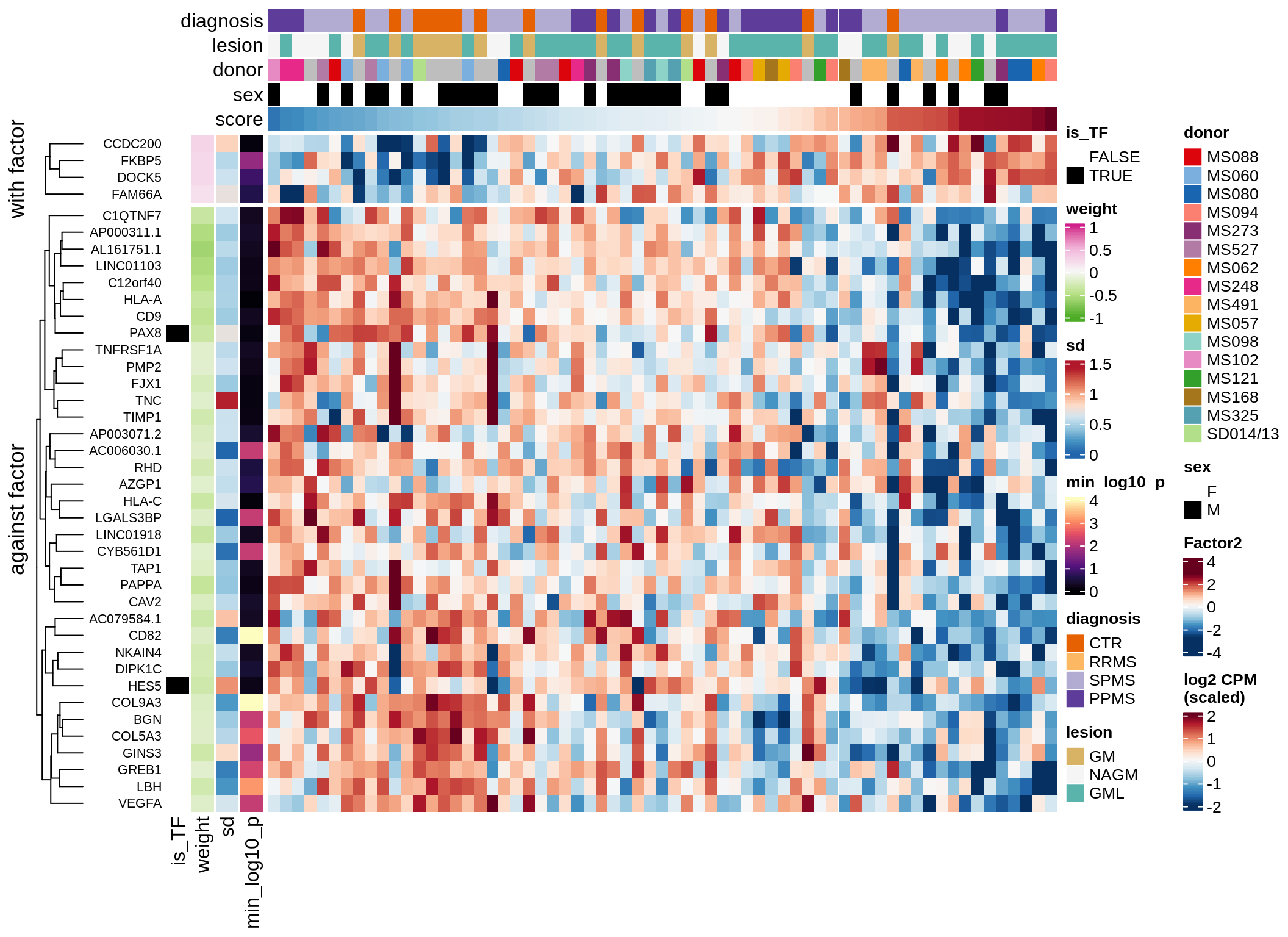
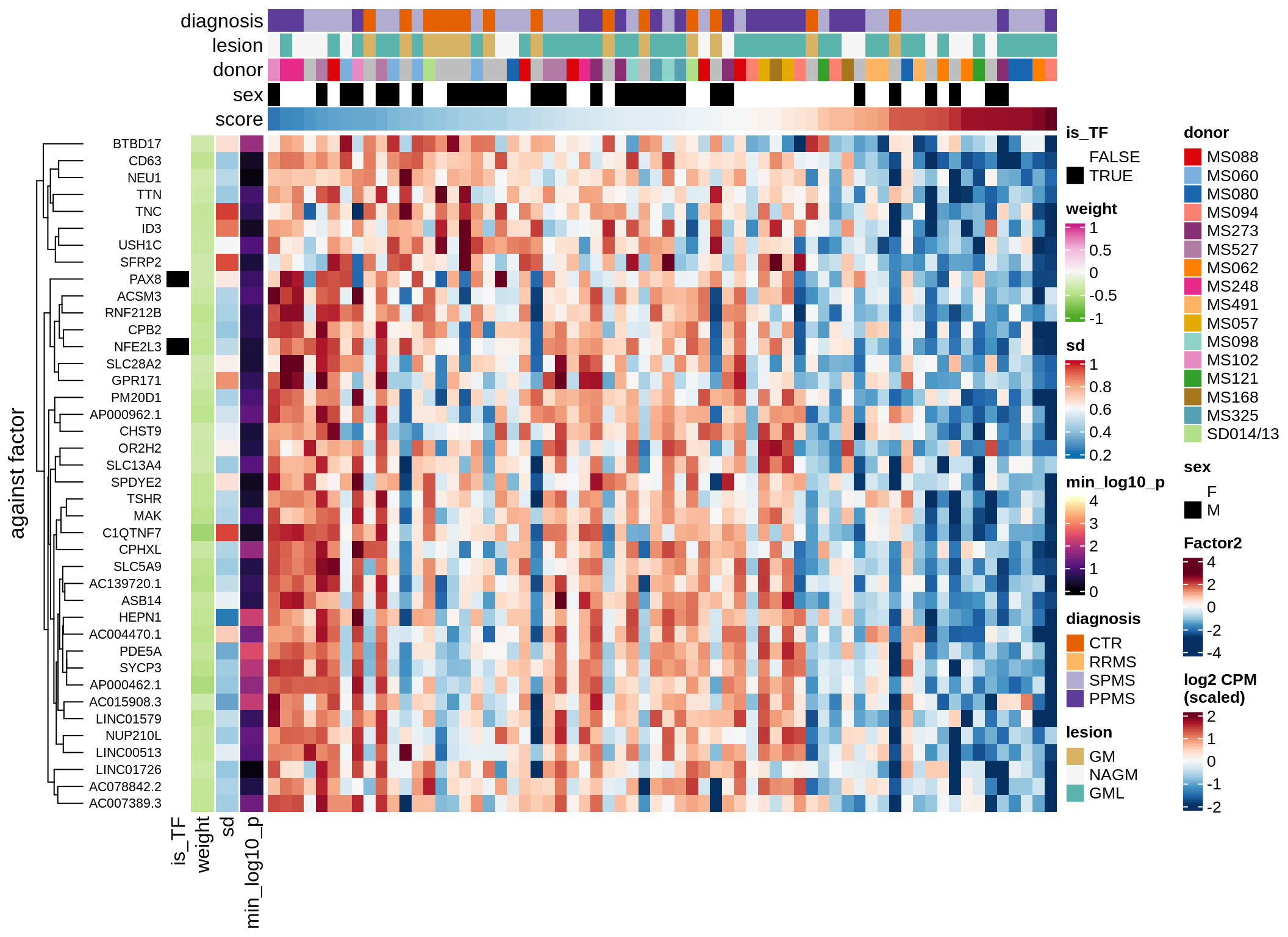
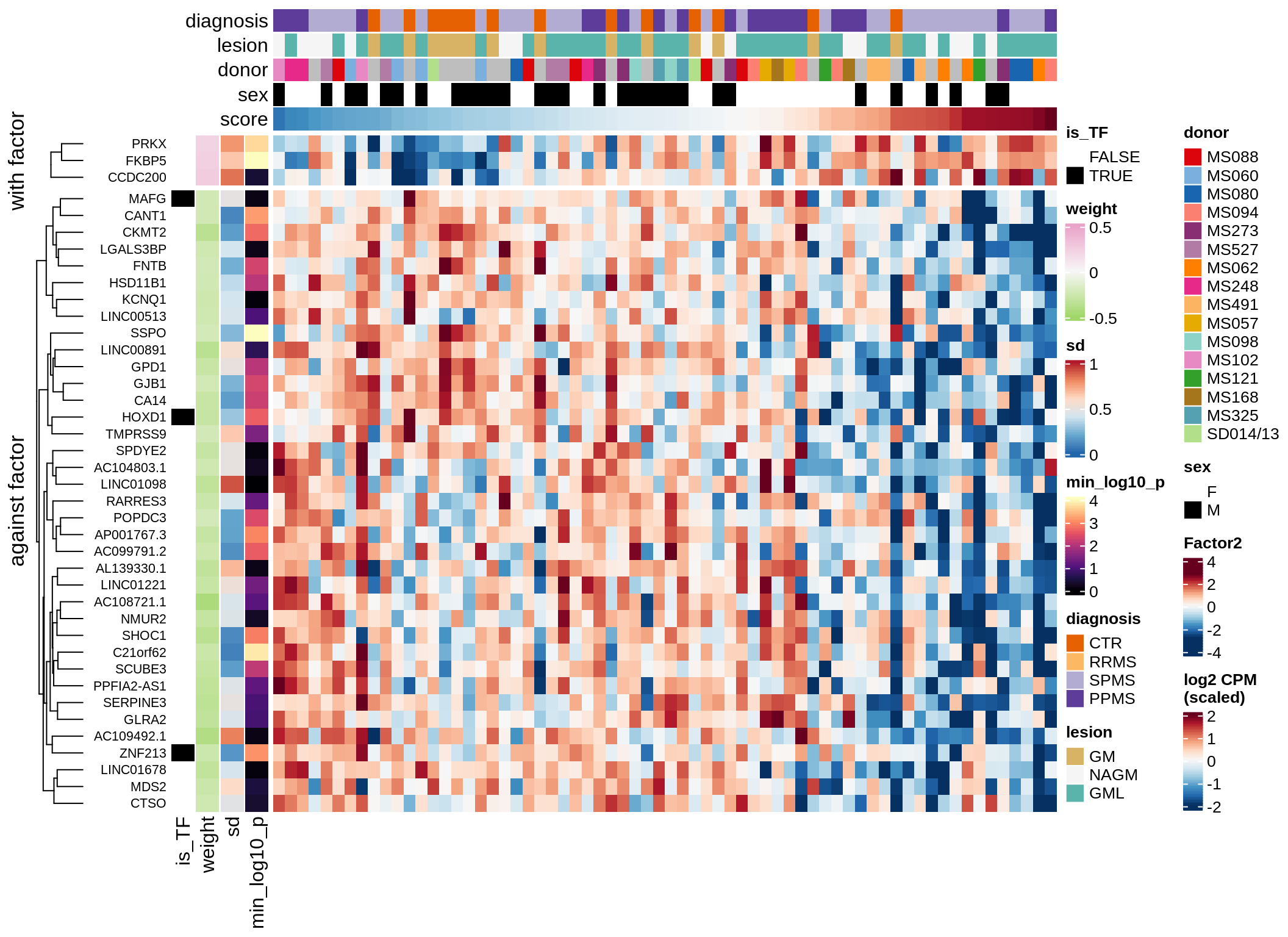
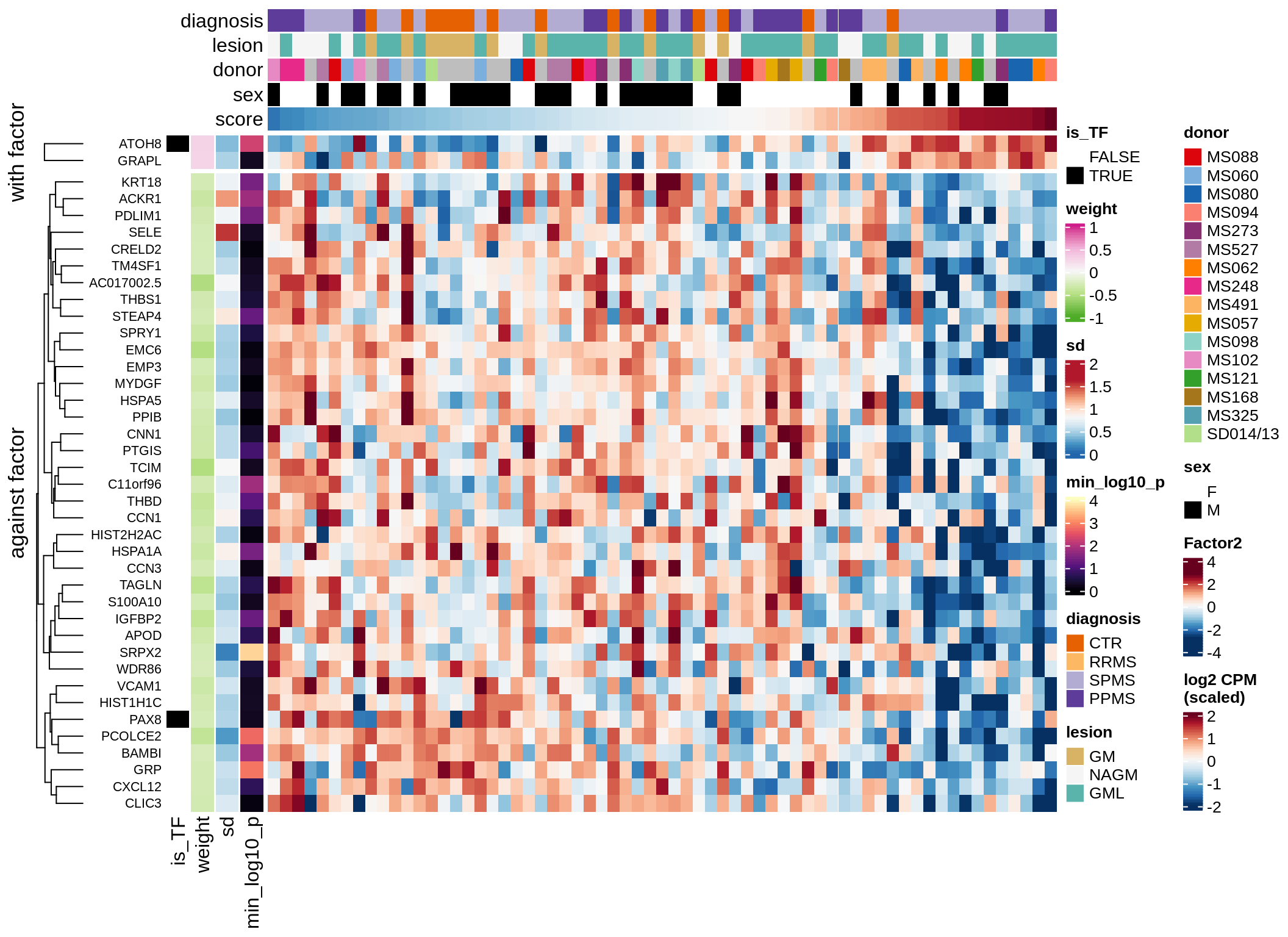
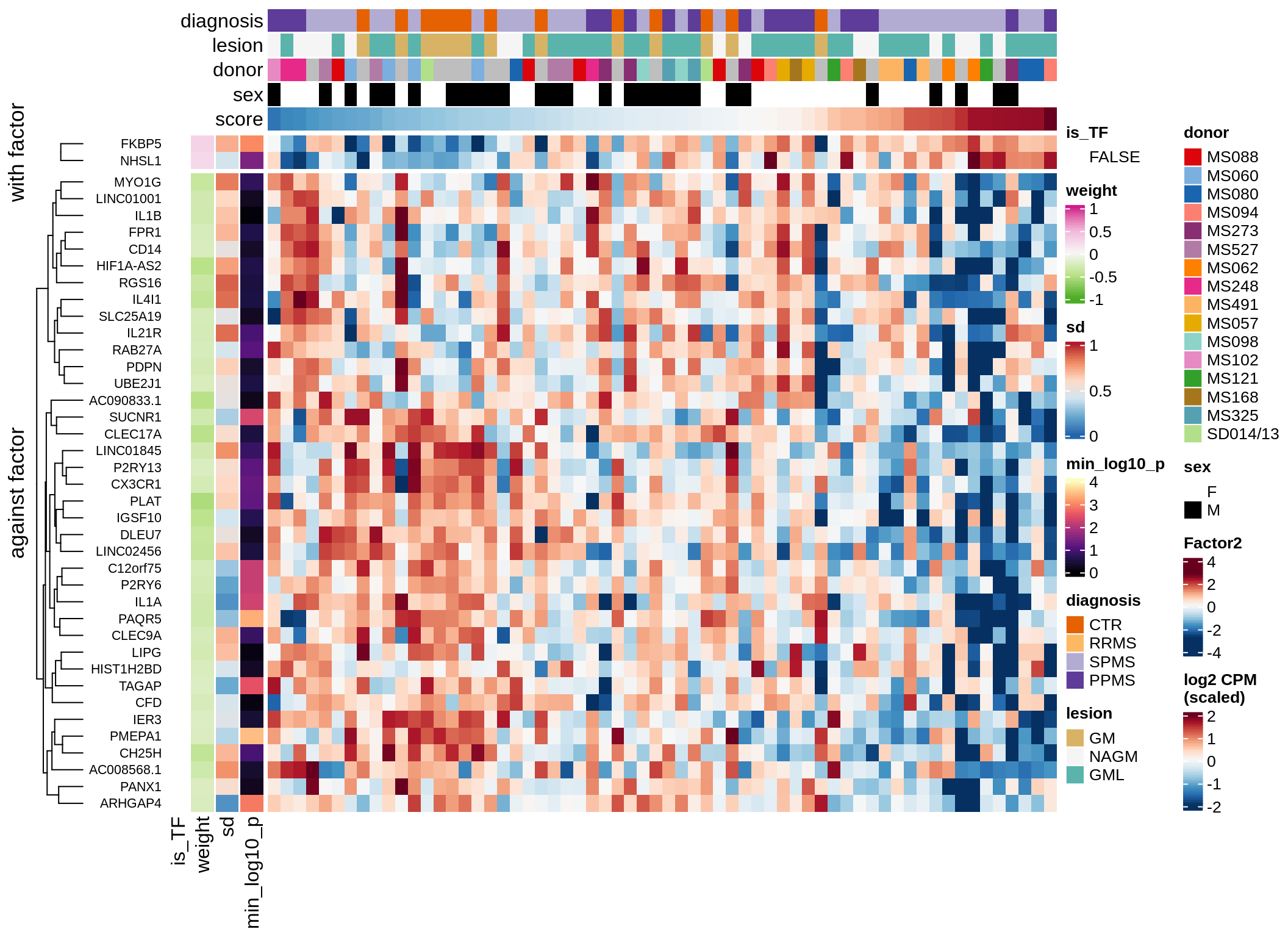
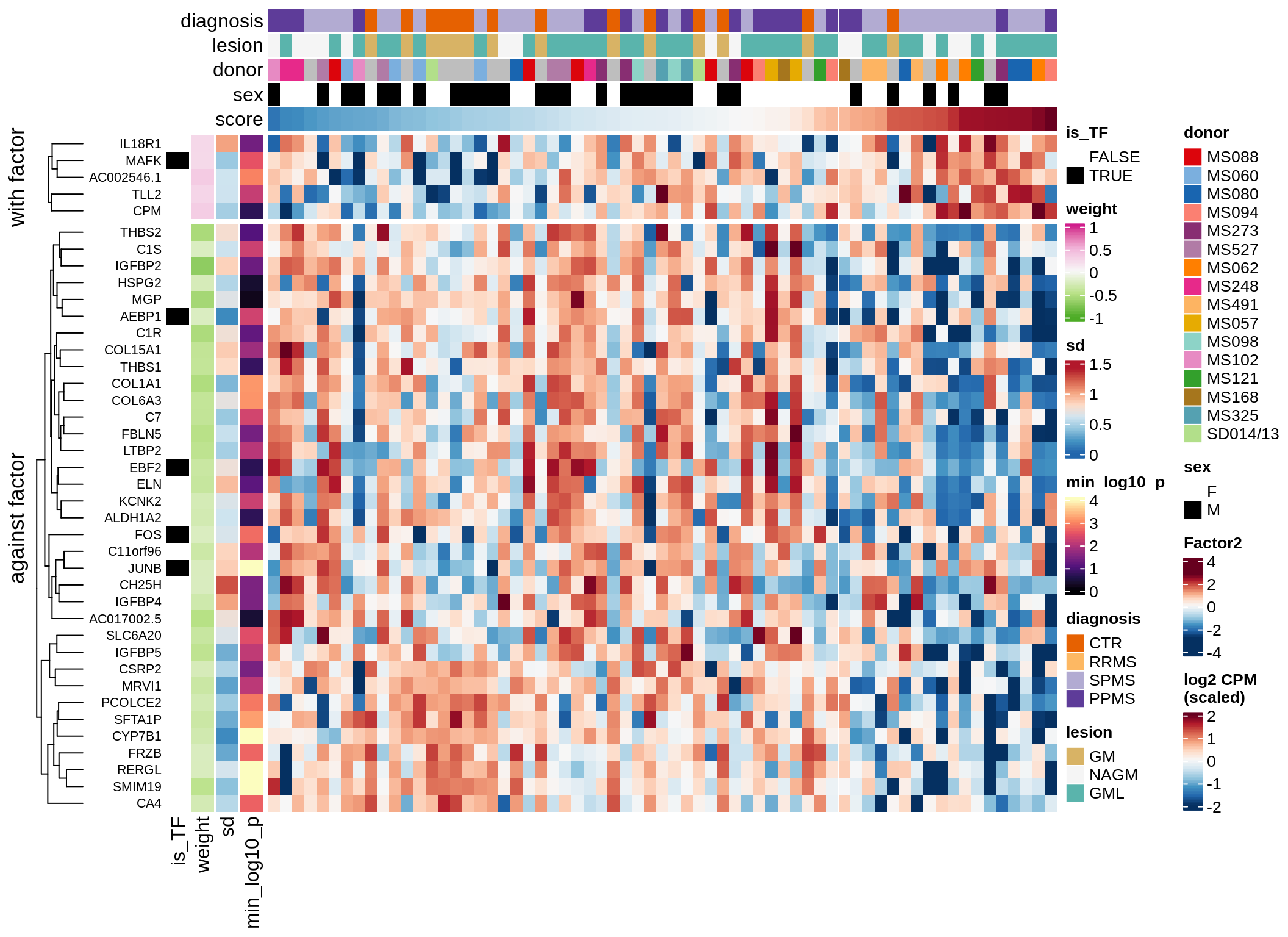
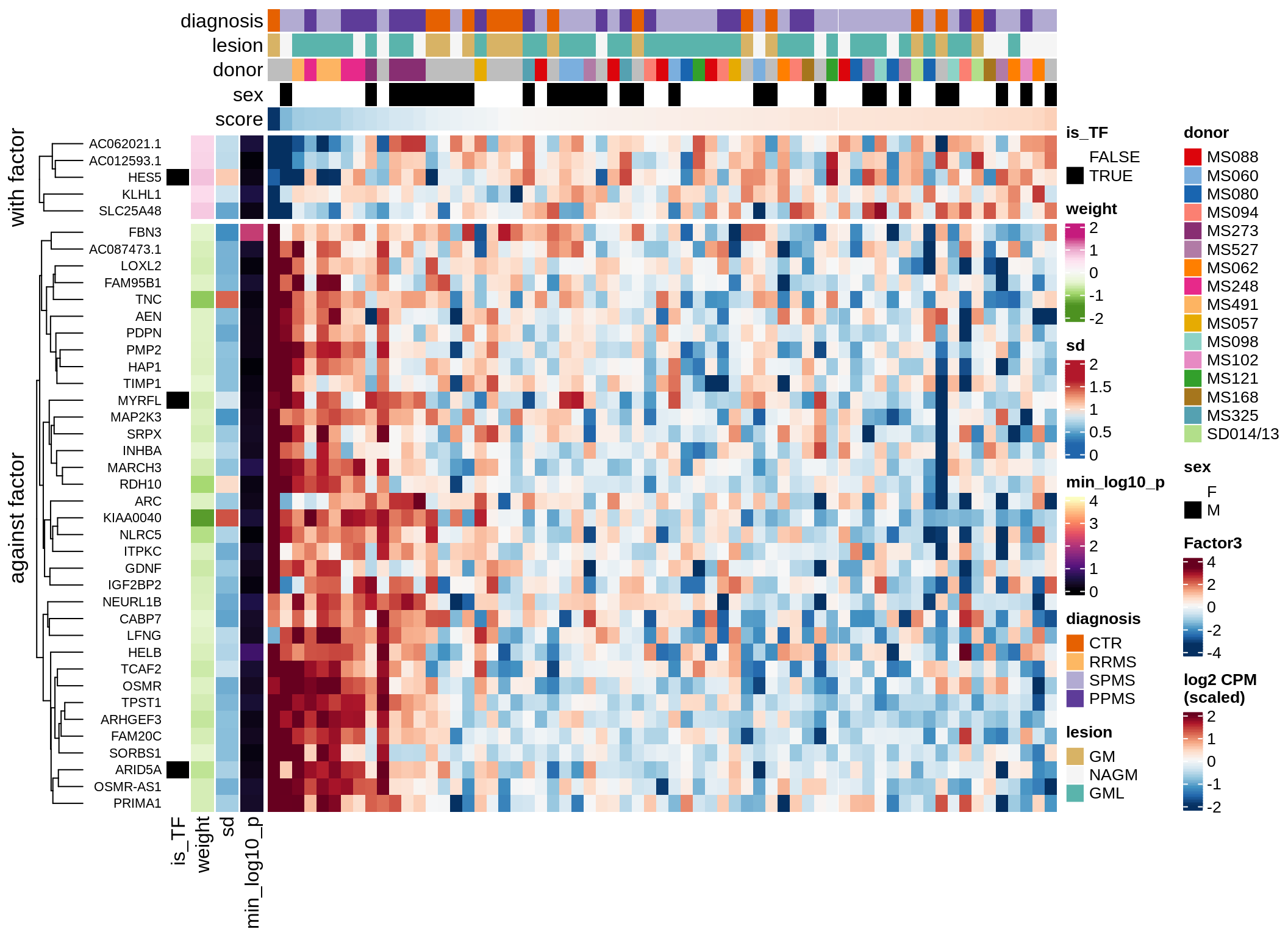
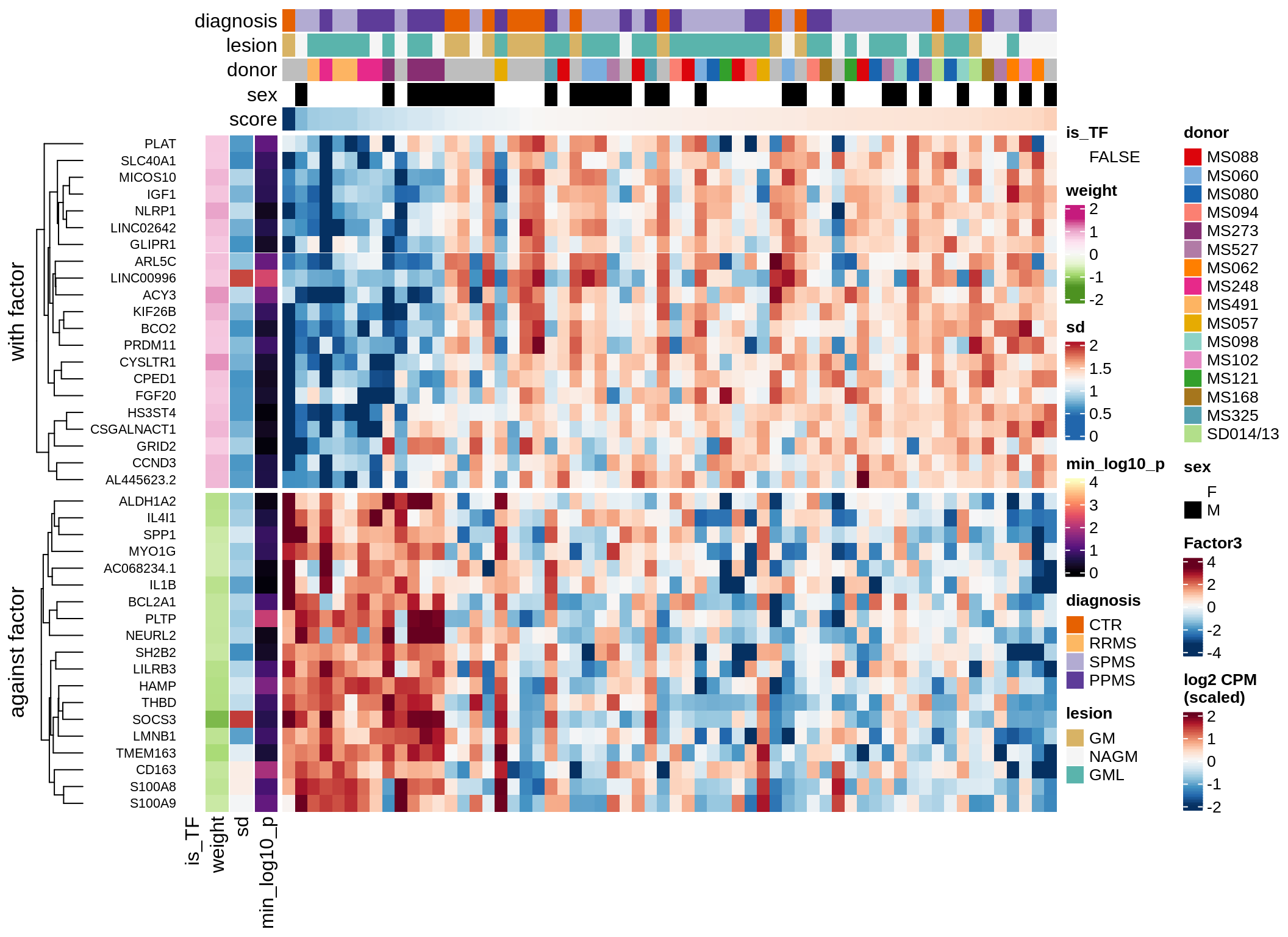
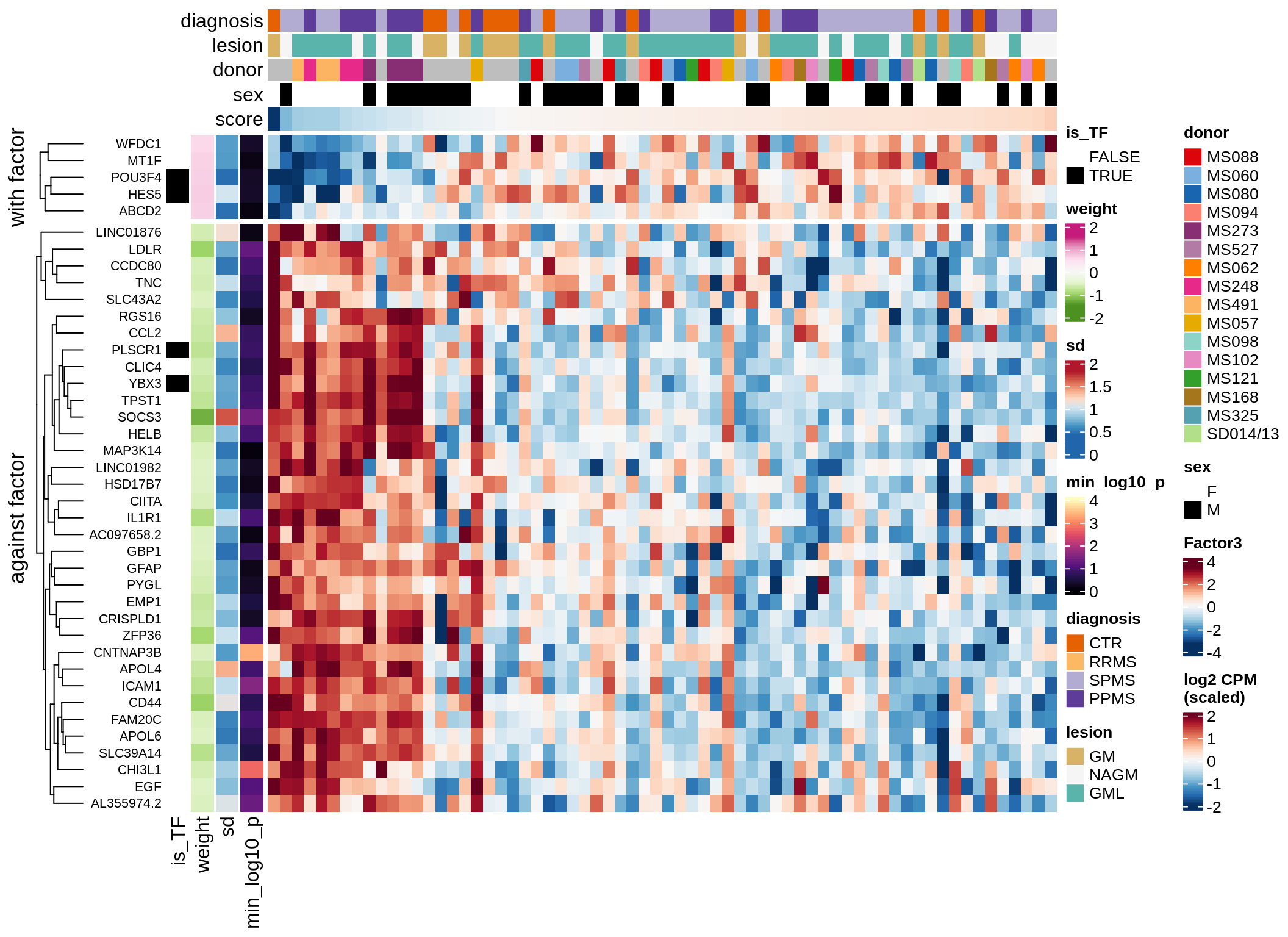
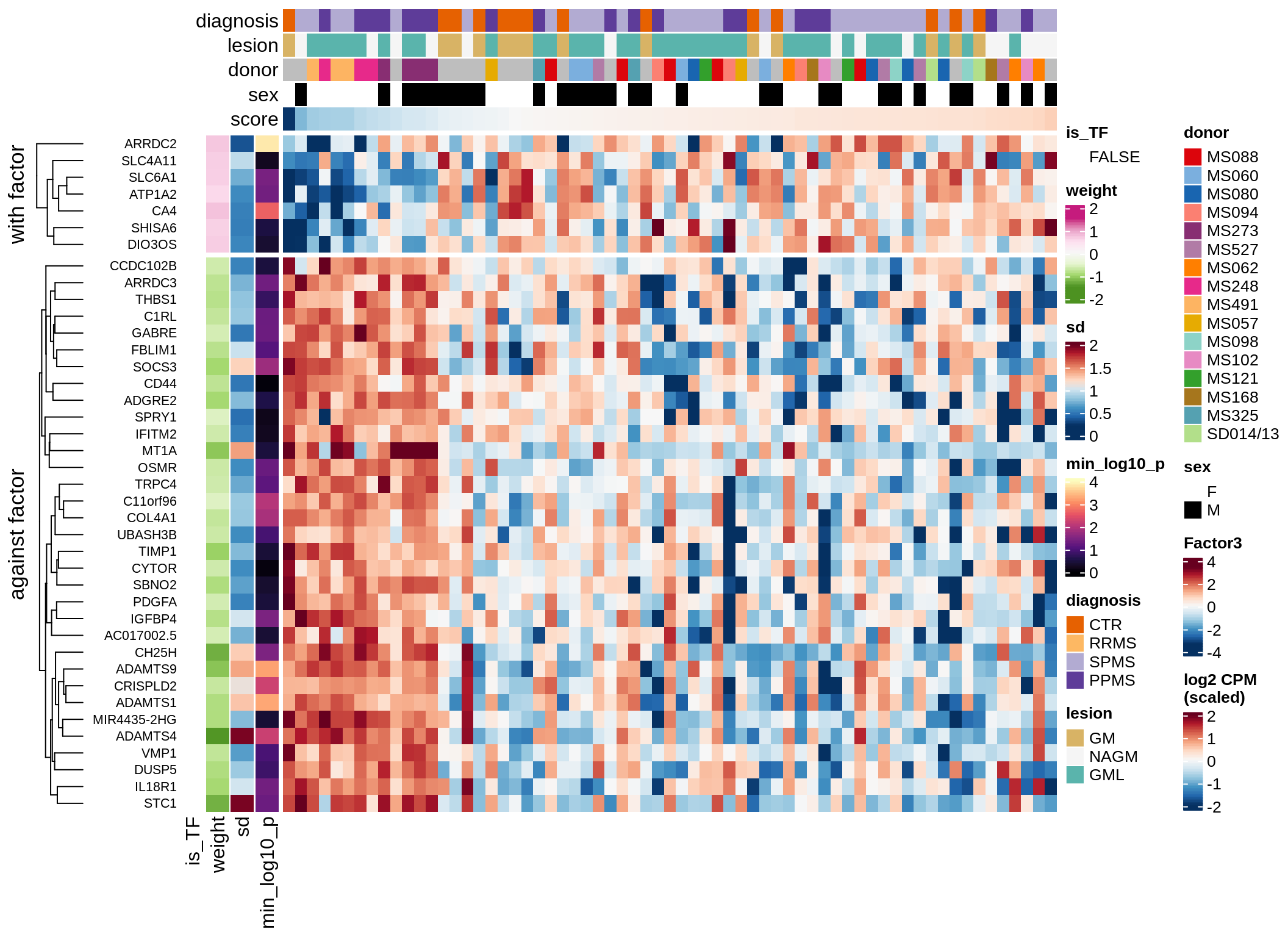
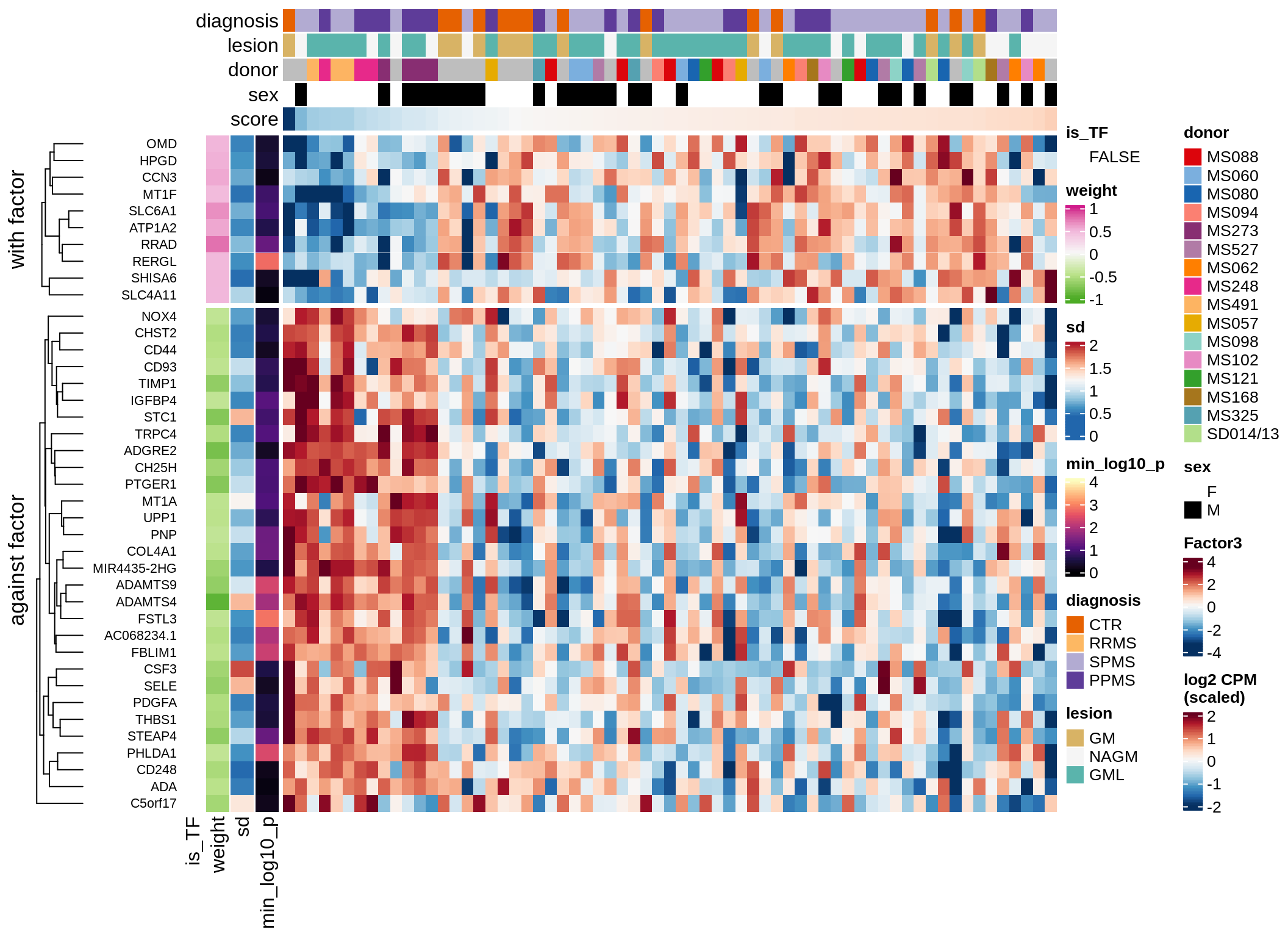
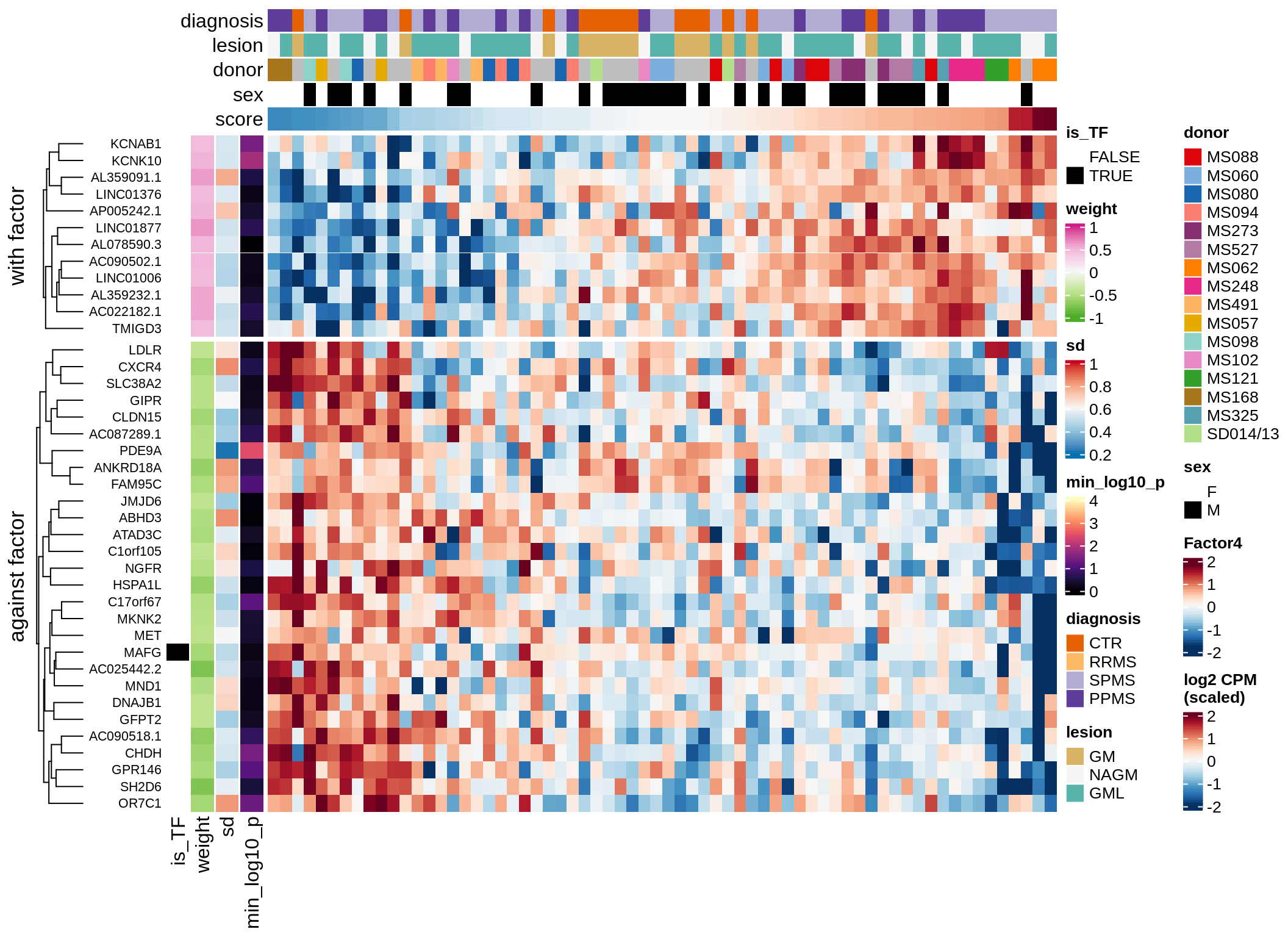
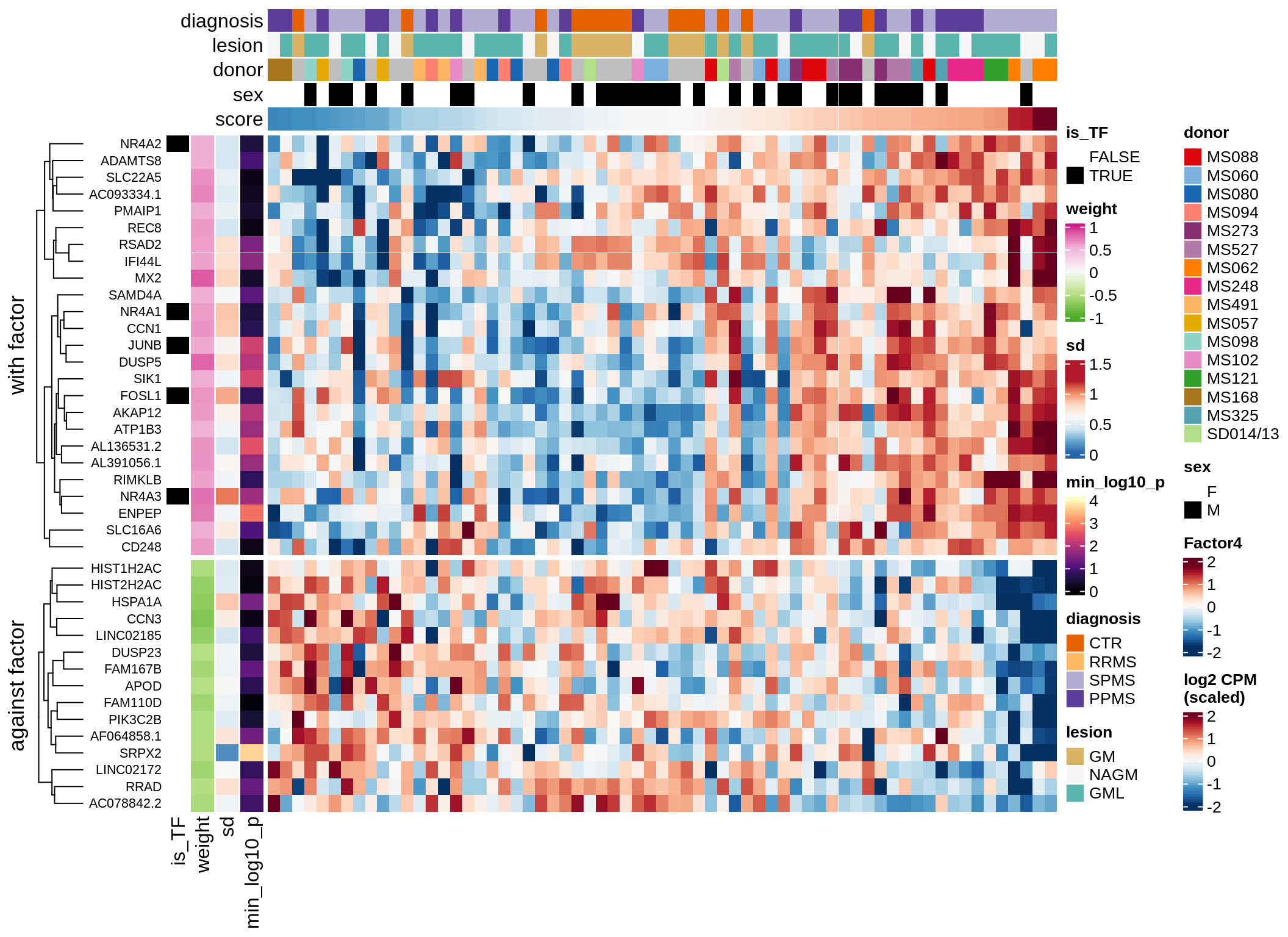
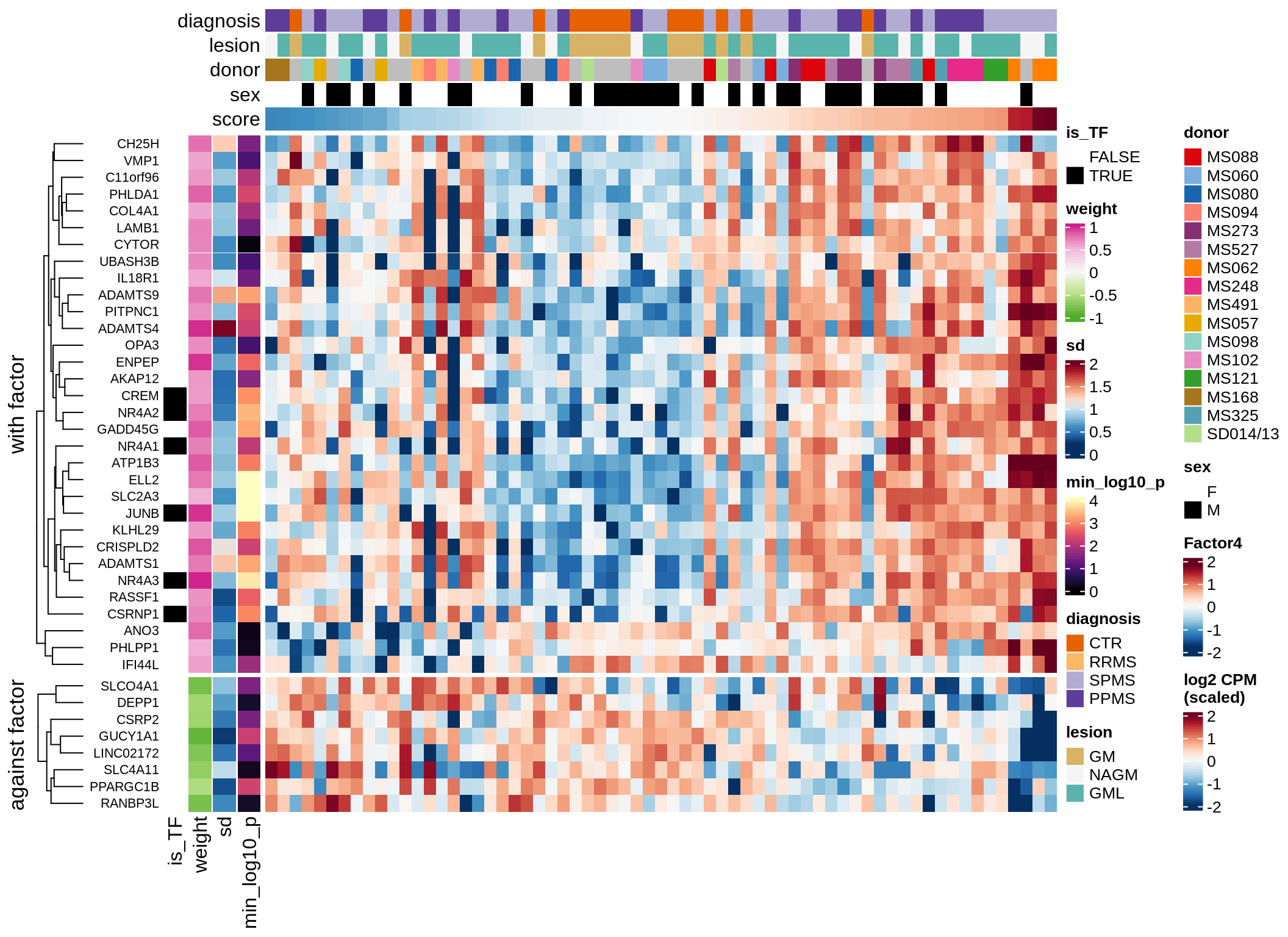
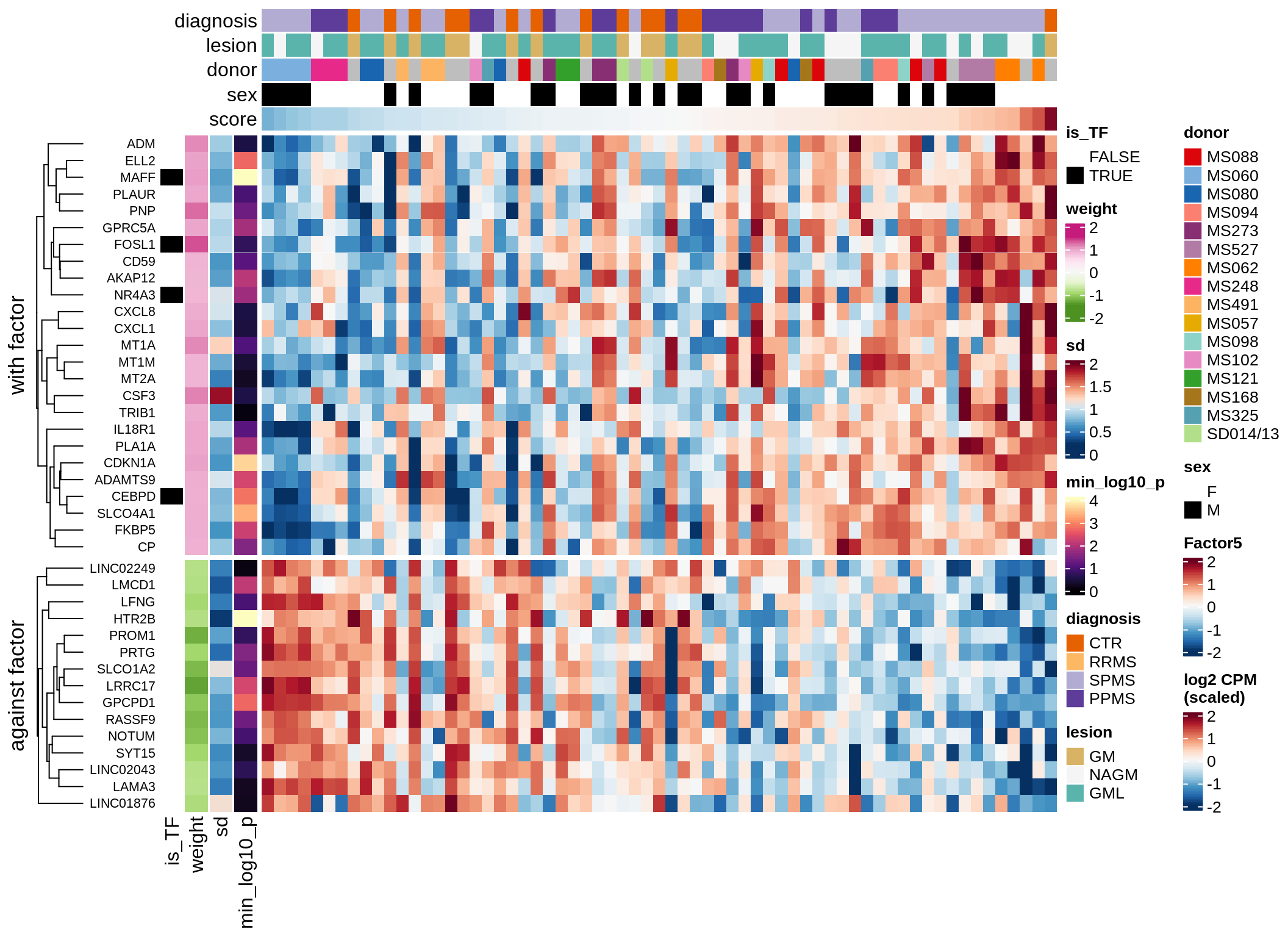
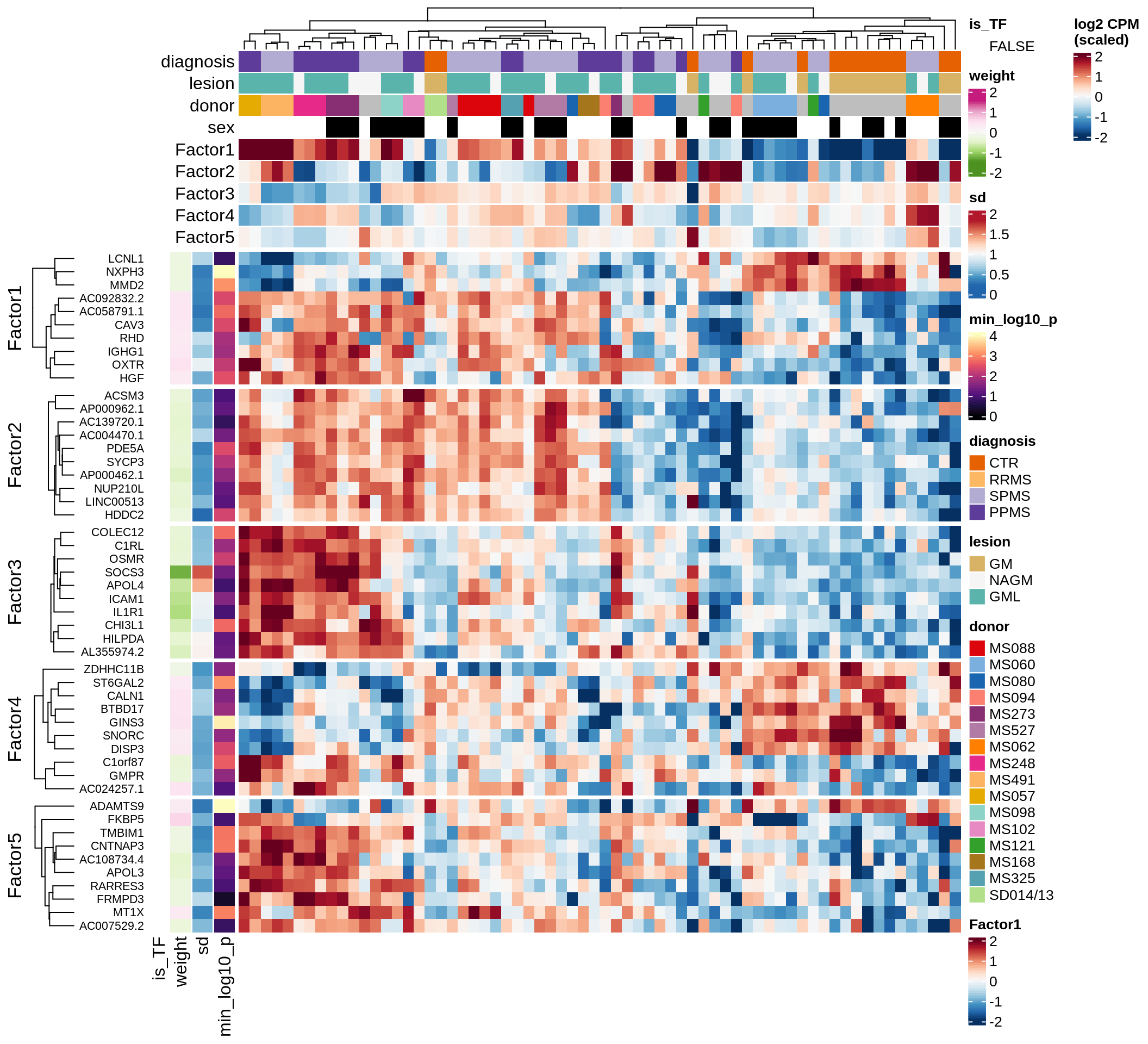
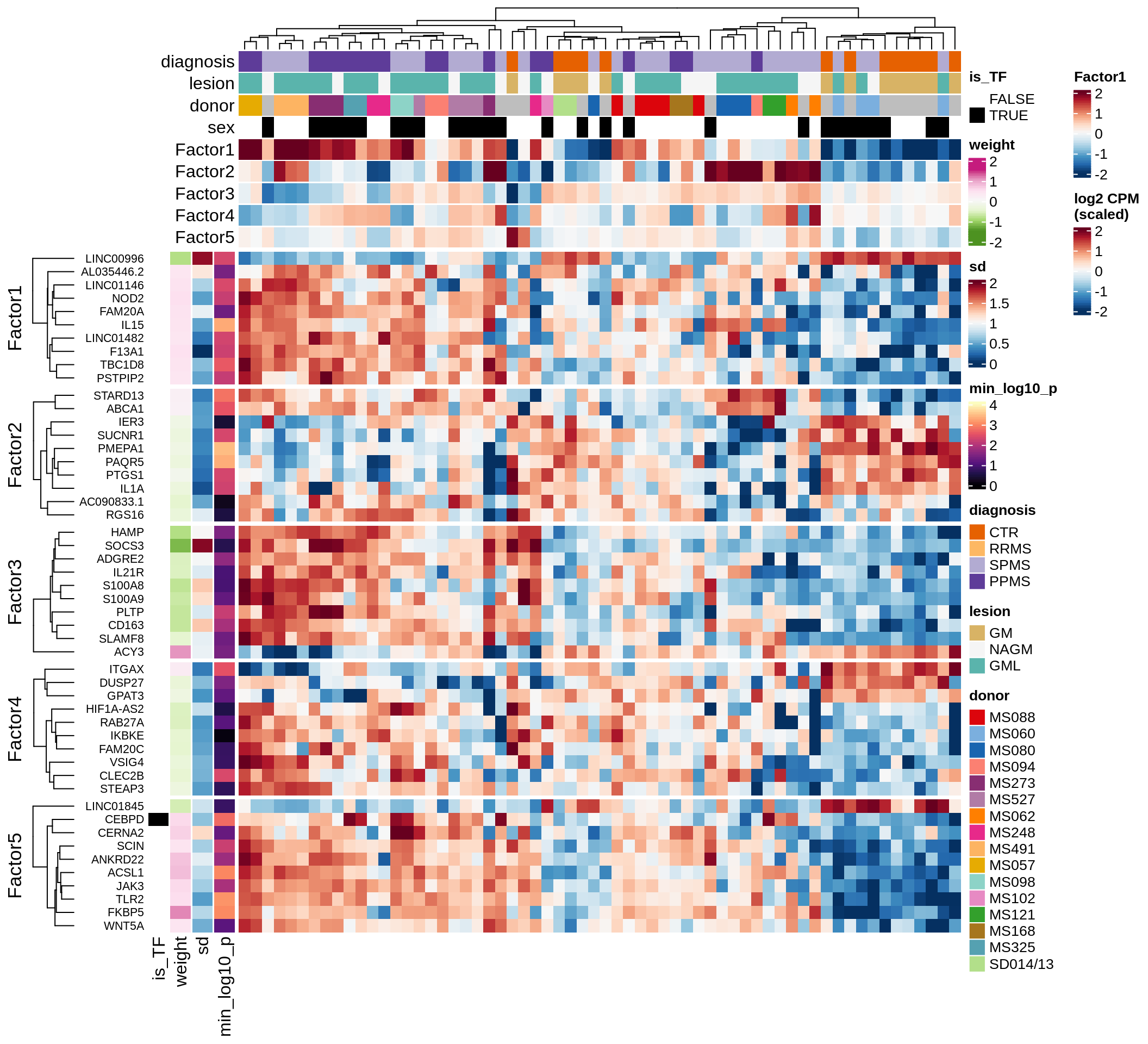
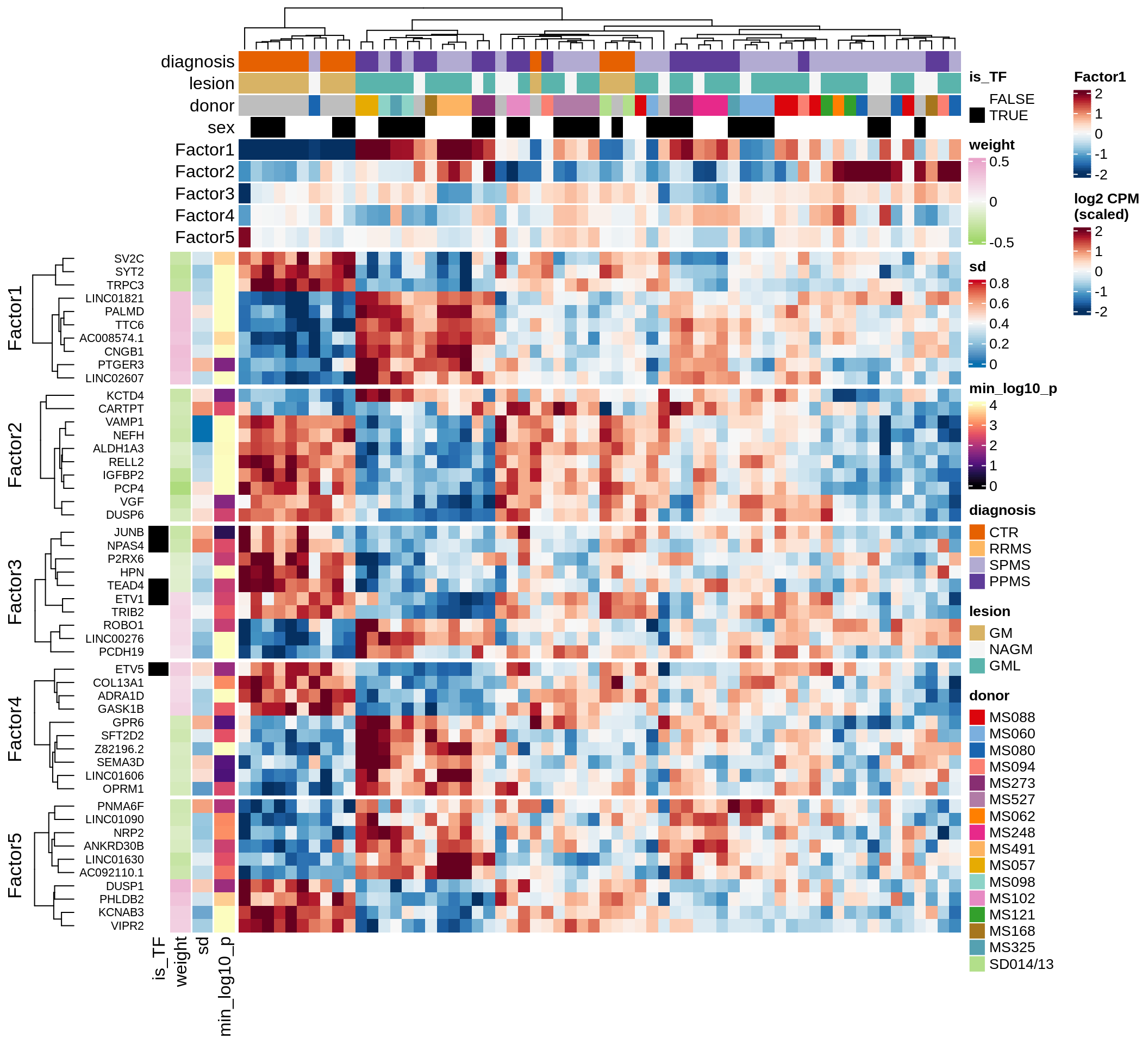
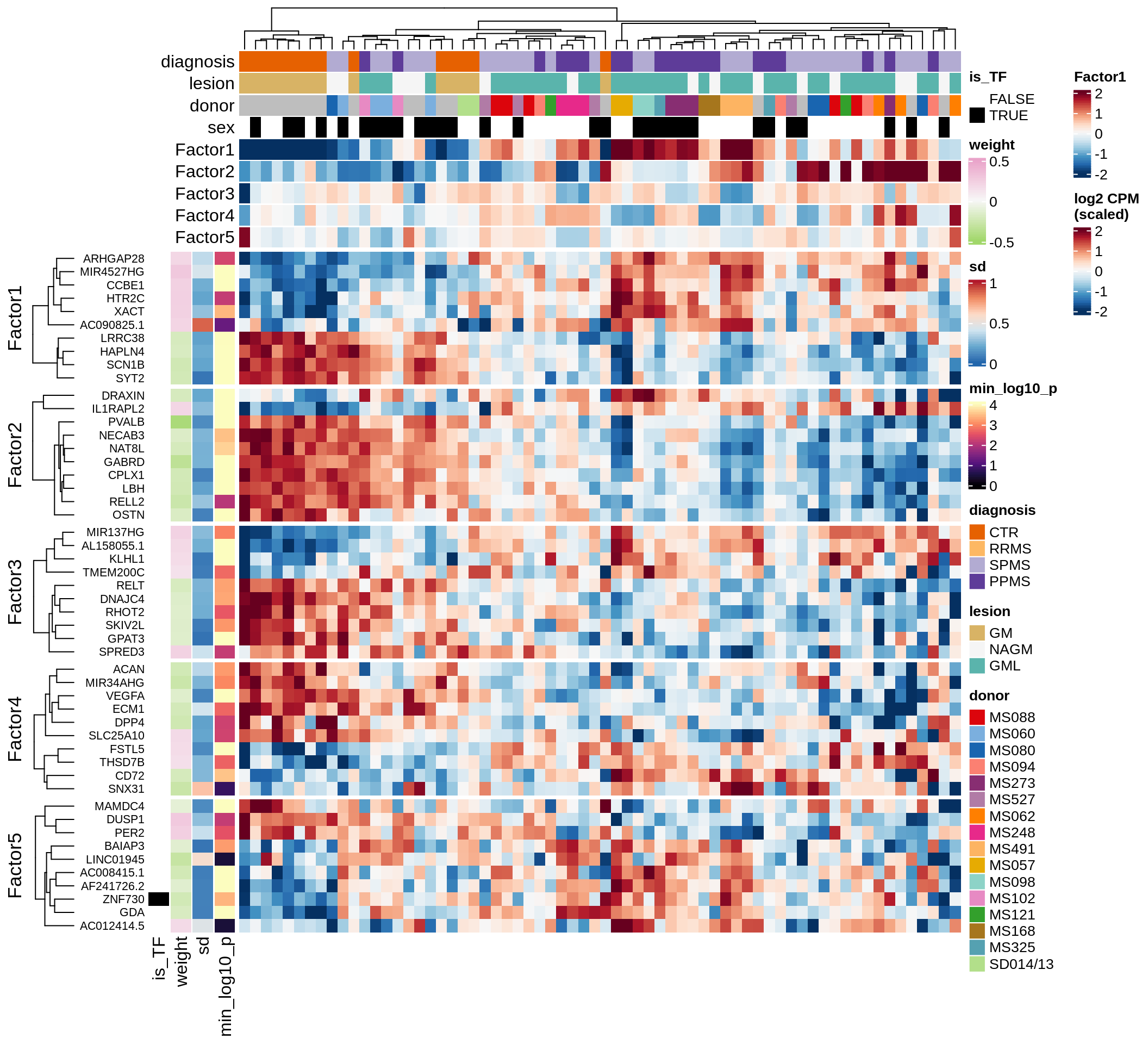
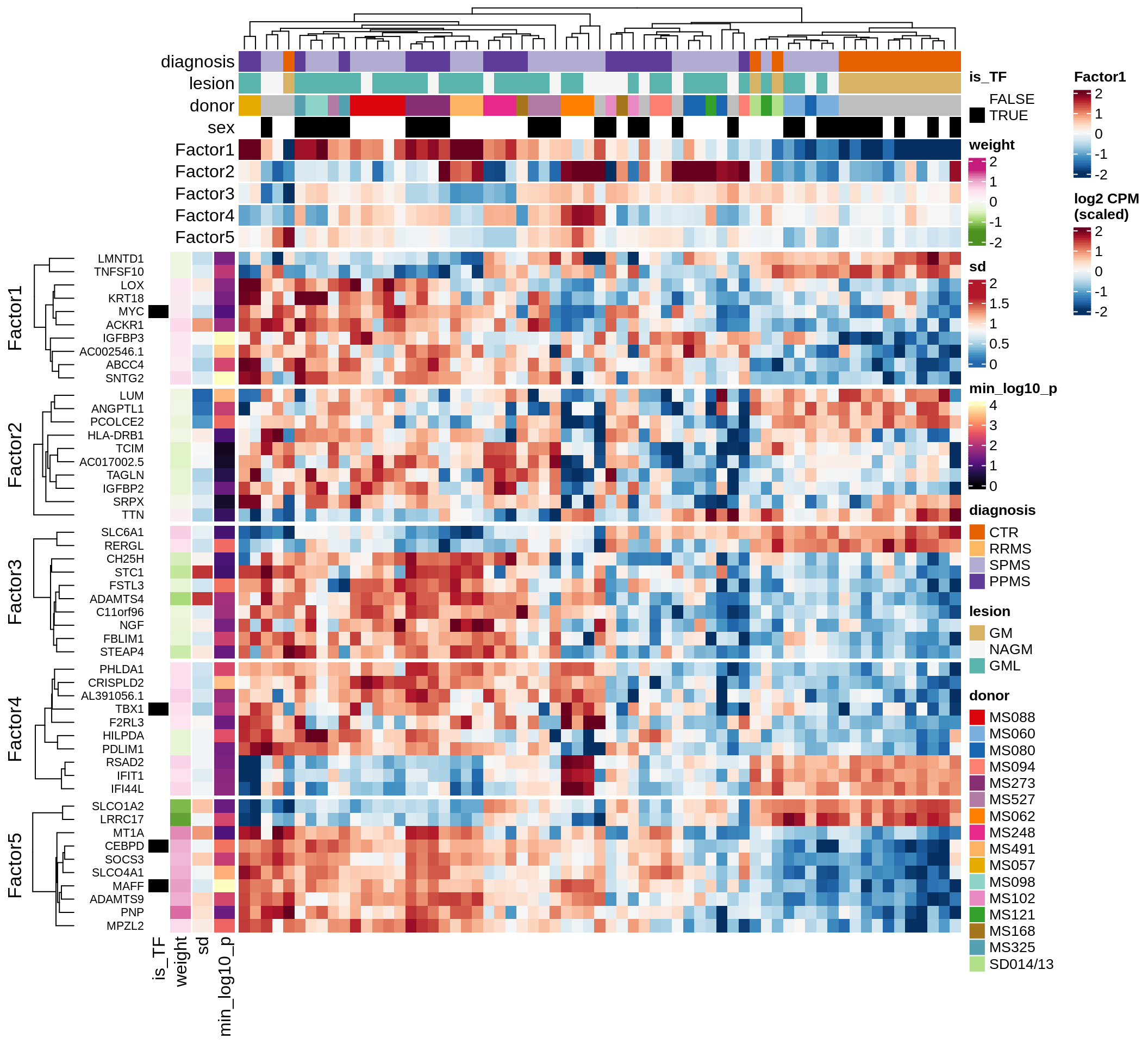
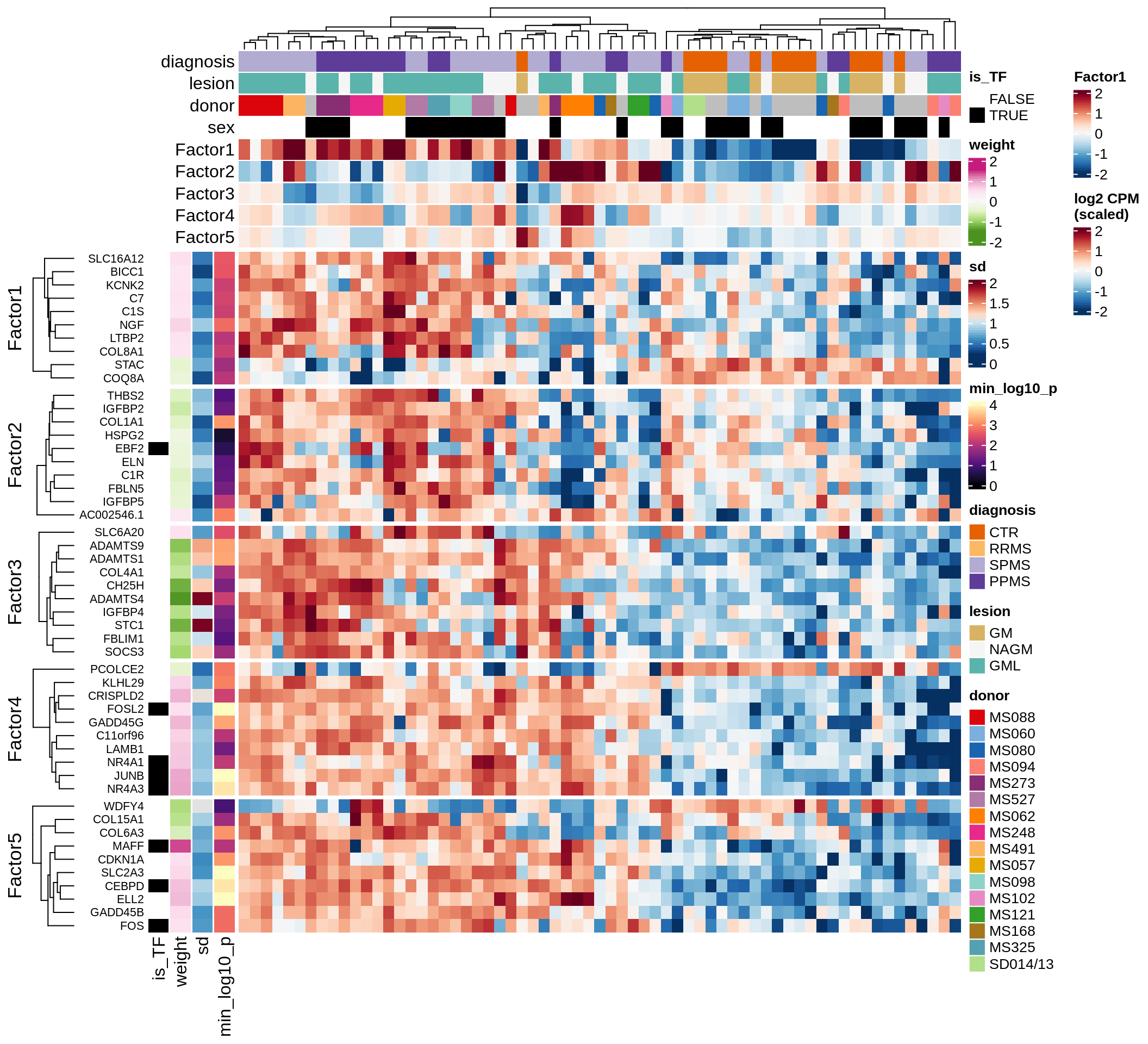
}Coefficients of top genes (by factor)
for (f in factors_names(model)) {
cat('### ', f, '\n', sep = '')
draw(plot_top_weights_heatmap_by_factor(model, var_exp_dt, sel_f = f))
cat('\n\n')
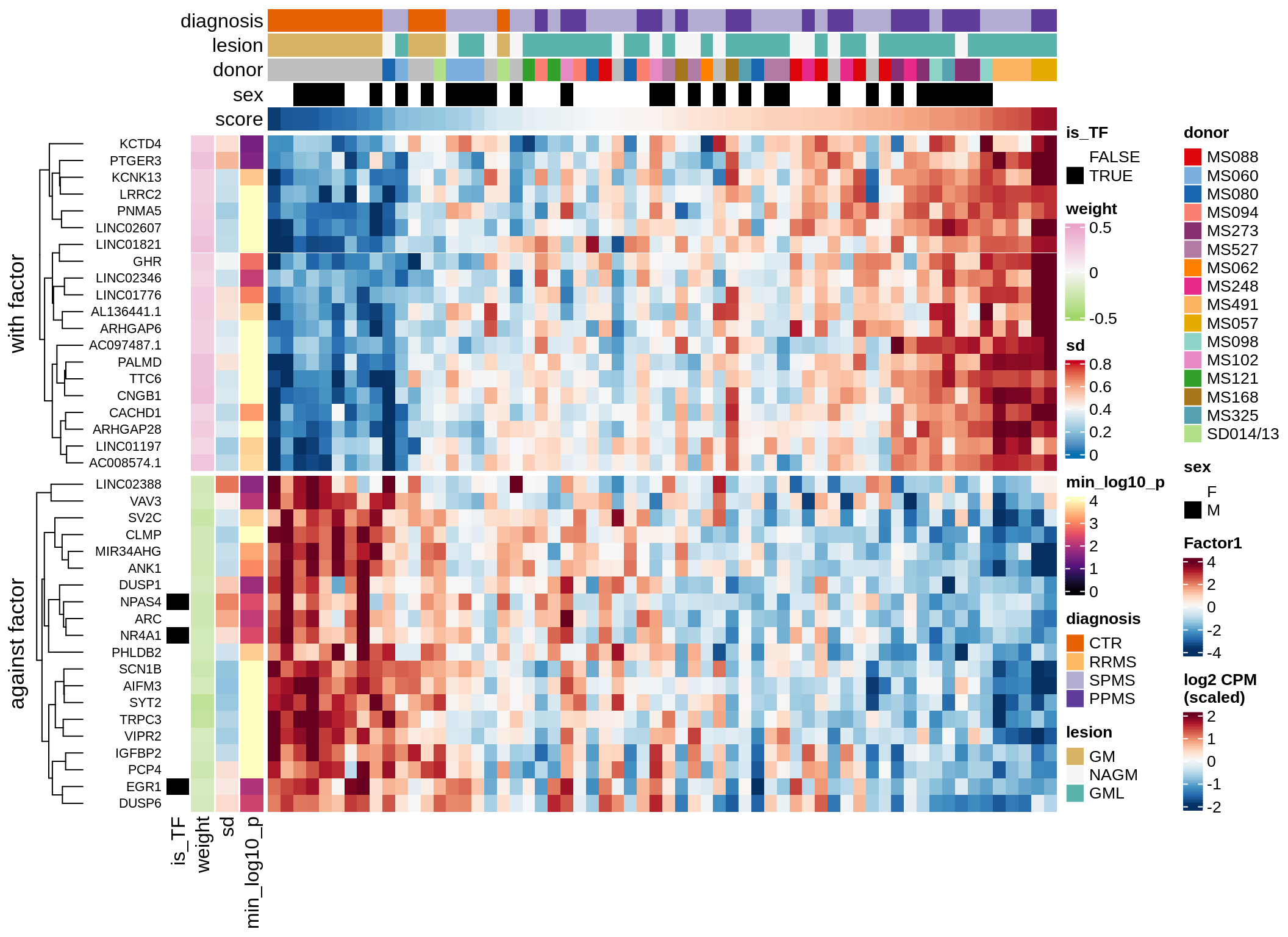
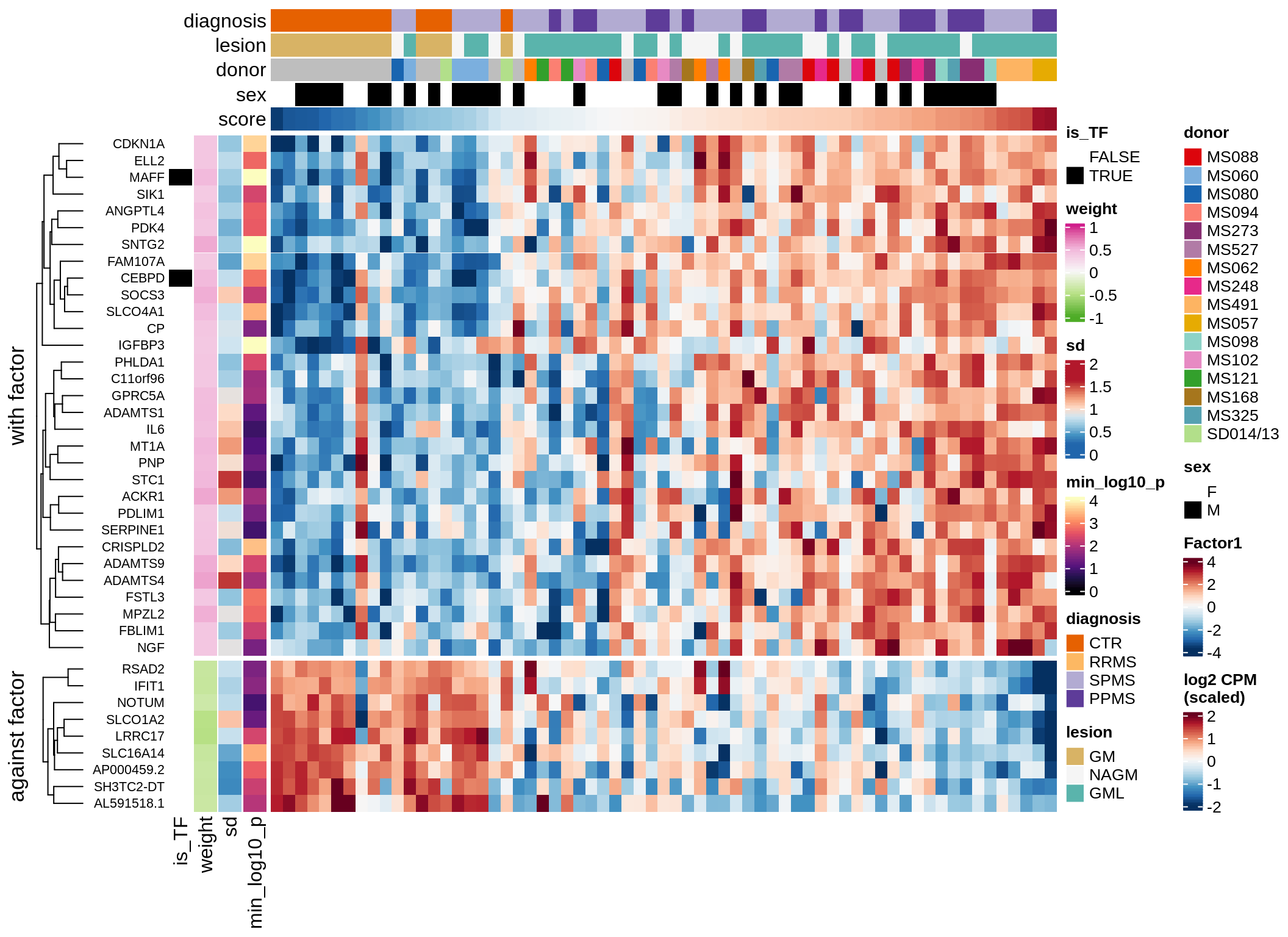
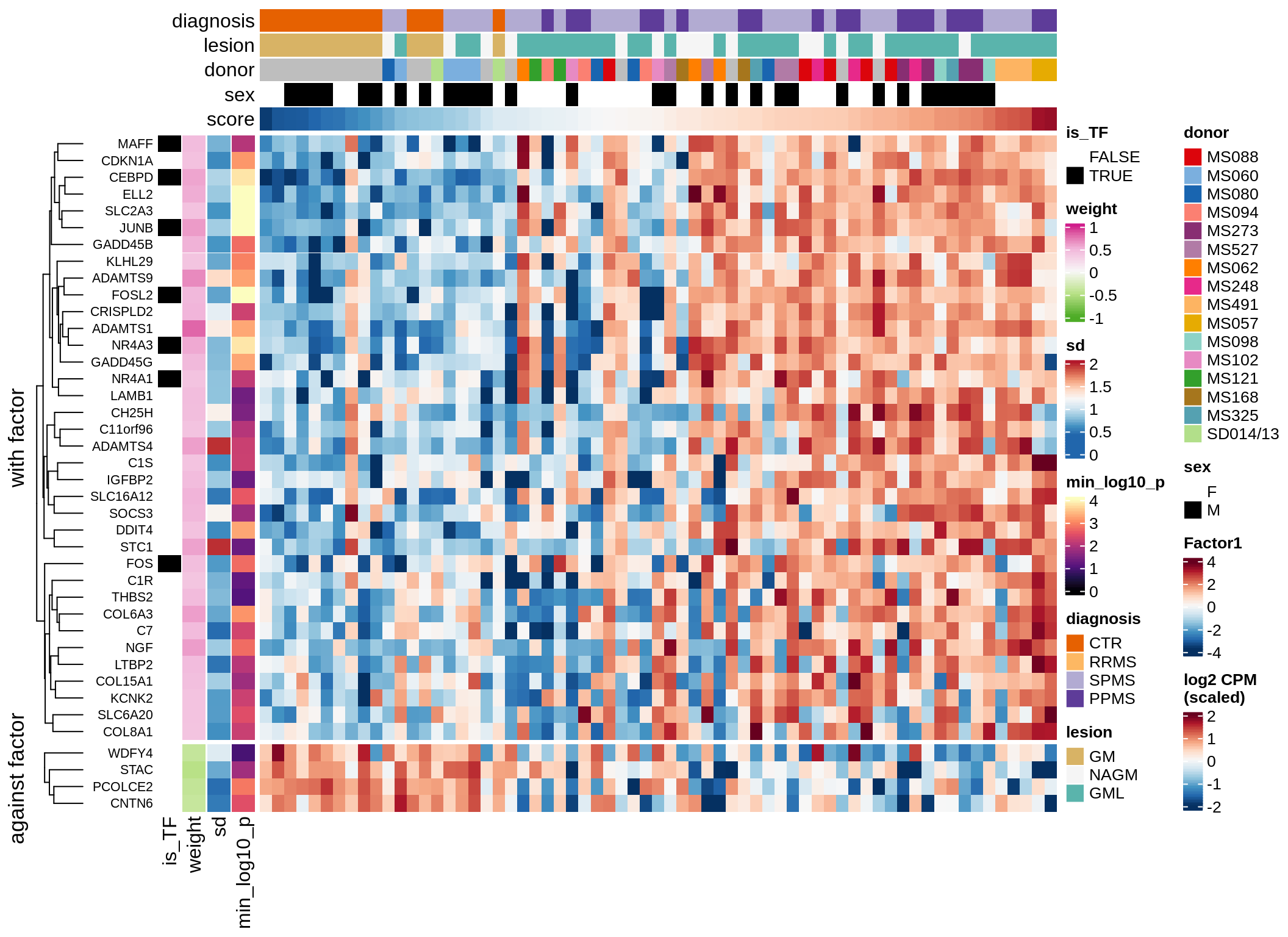
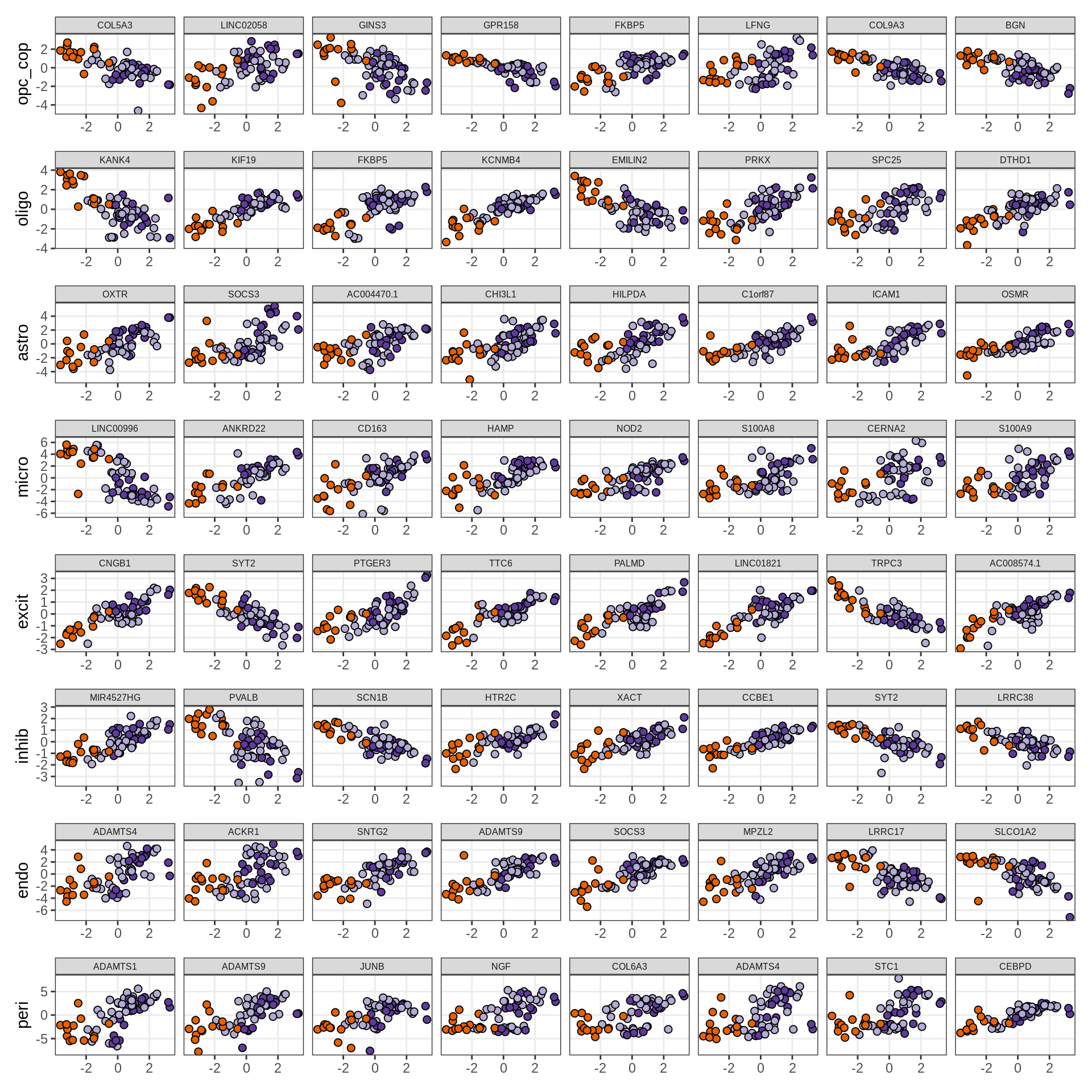
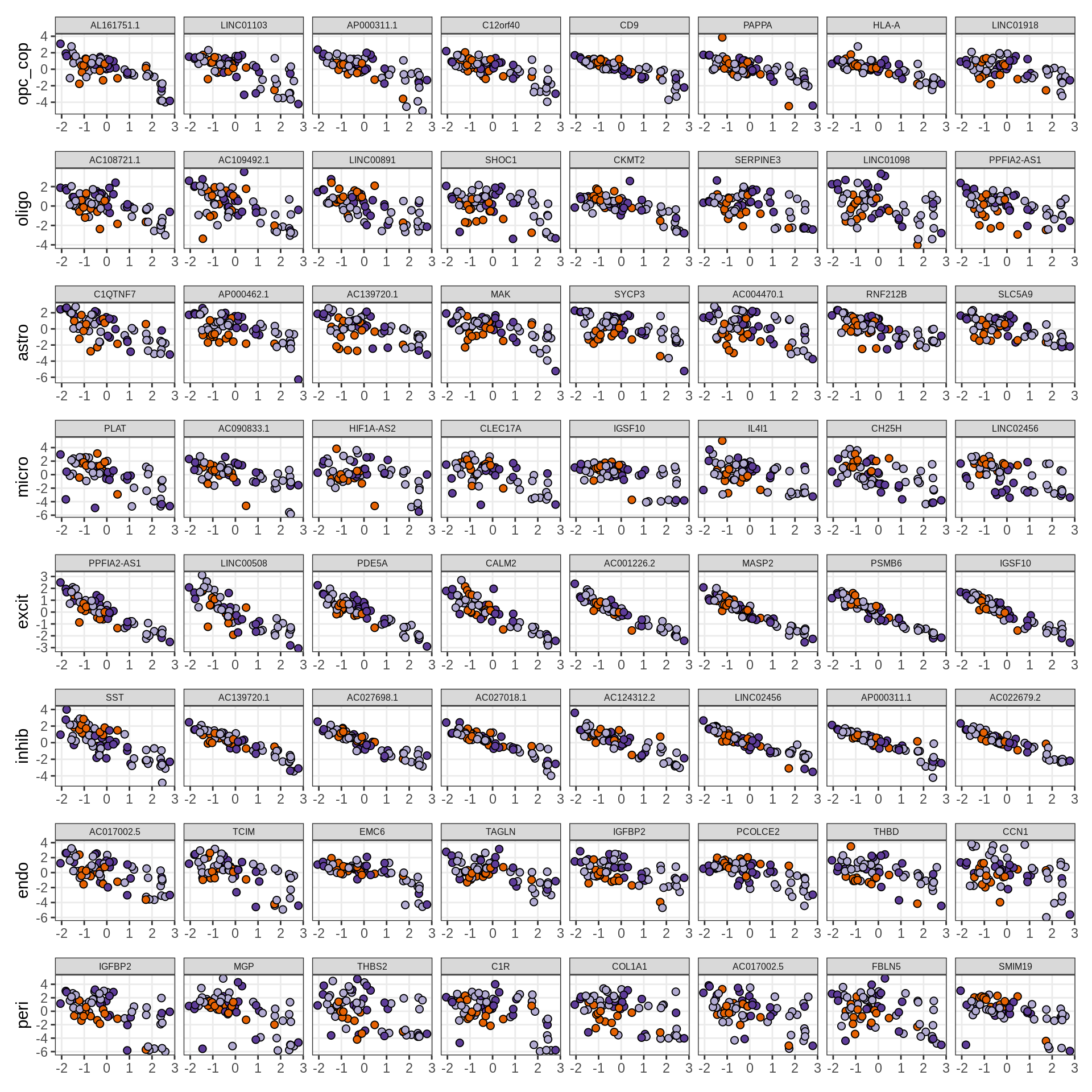
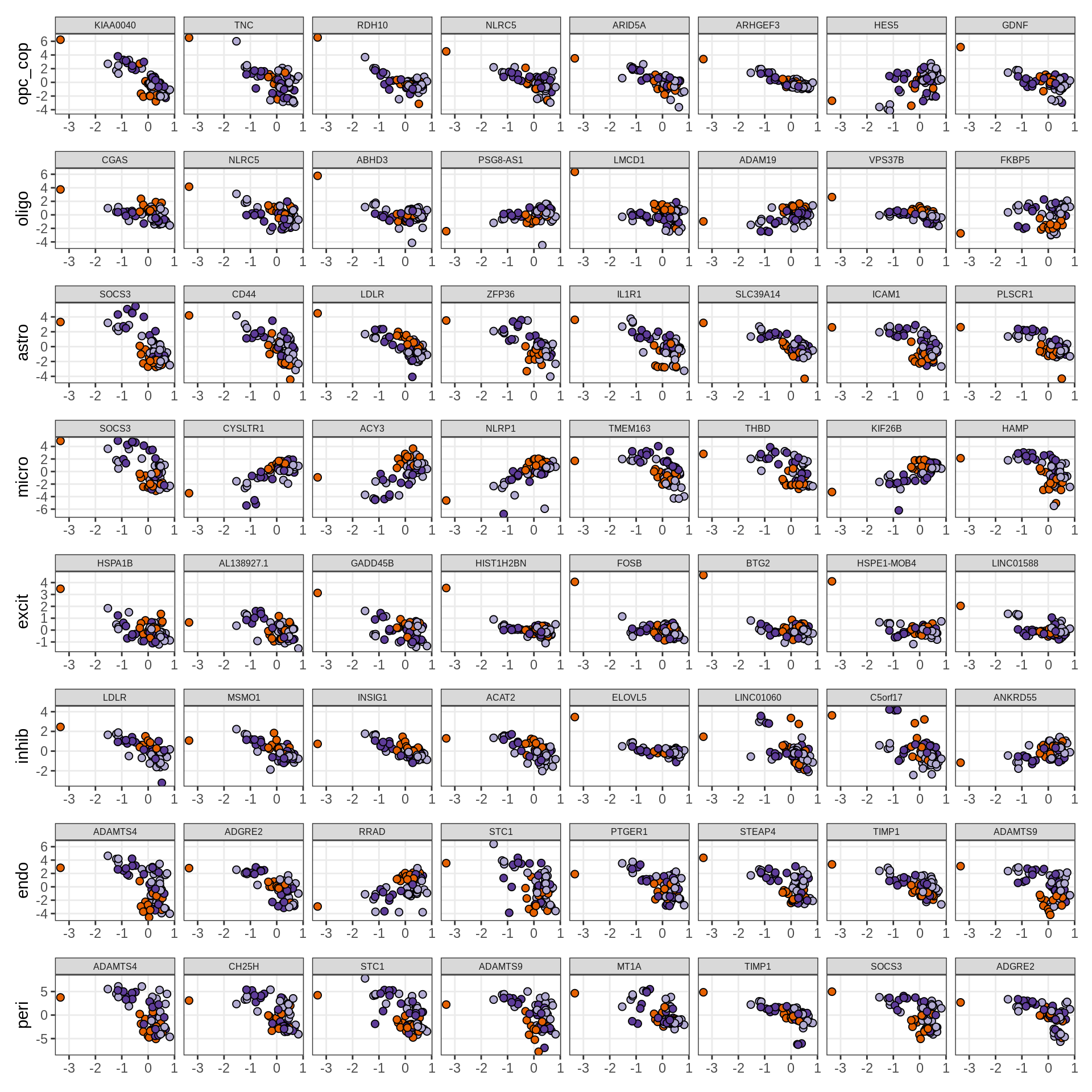
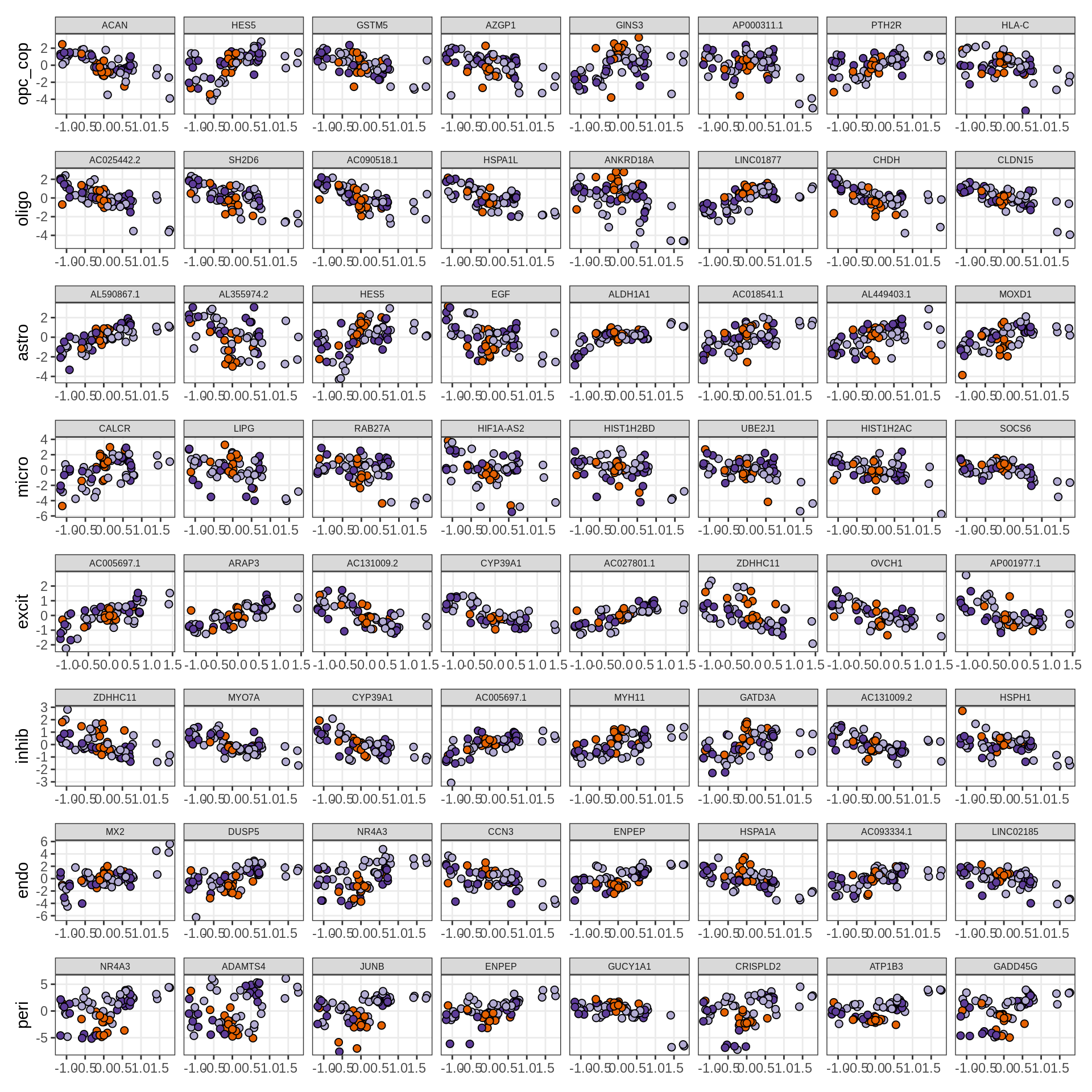
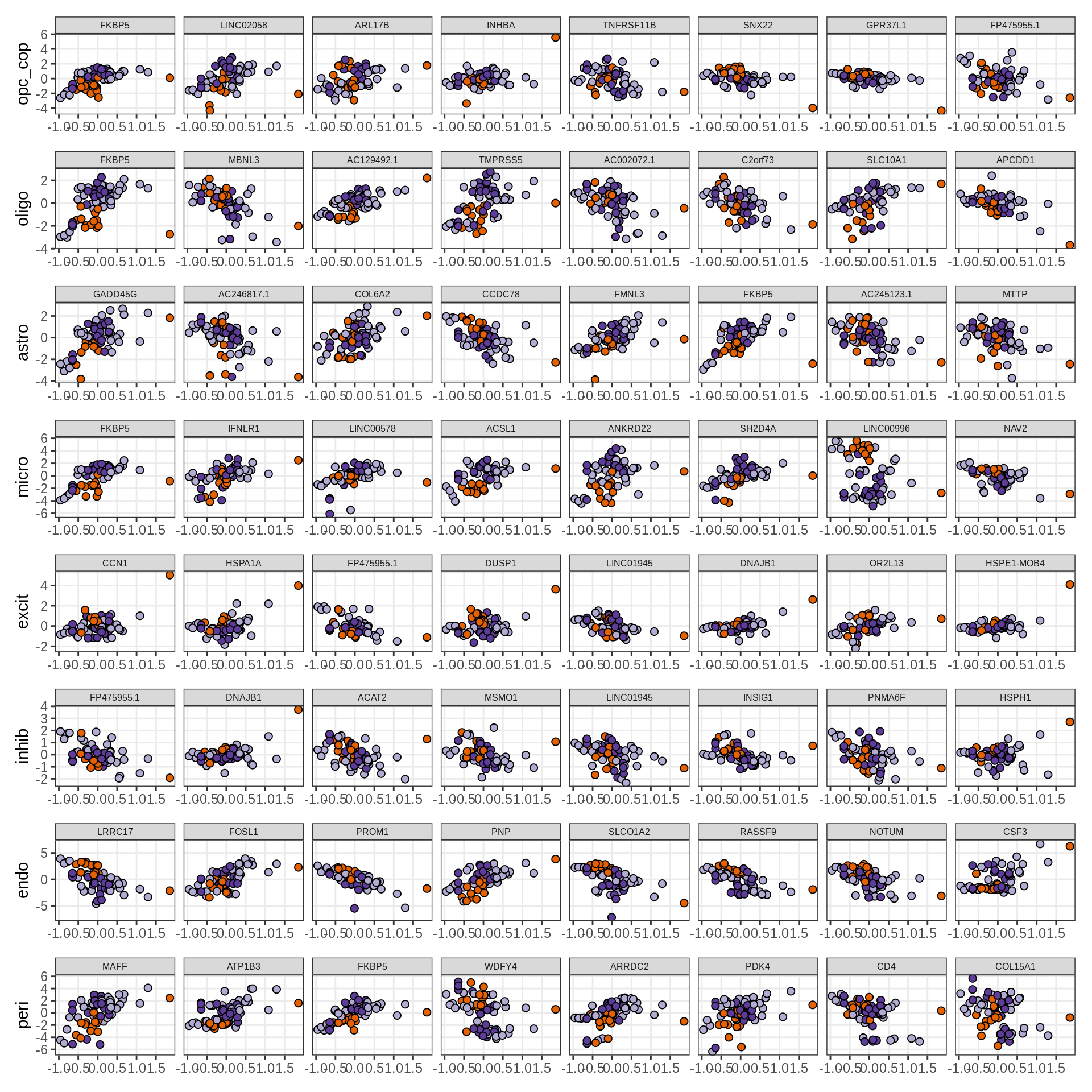
}Expression of top genes per celltype
# iterate plots
for (i in seq.int(nrow(to_plot_dt))) {
sel_v = as.character(to_plot_dt[i]$view)
sel_f = to_plot_dt[i]$factor
this_r2 = to_plot_dt[i]$var_exp
cat('### ', sel_v, '-F', as.integer(sel_f),
' (', round(this_r2, 0), '%)', '\n', sep = '')
draw(plot_top_weights_expression_sample(model, pb, annots_dt, filter_dt,
tfs_dt, sel_v = sel_v, sel_f = sel_f, n_top = 40, is_regressed = TRUE),
merge_legend = TRUE )
cat('\n\n')
}Expression of top genes across all factors per celltype
# iterate plots
for (sel_v in broad_short[sel_cl]) {
cat('### ', sel_v, '\n', sep = '')
draw(plot_top_genes_expression_all_factors(model, pb, annots_dt, filter_dt,
tfs_dt, var_exp_dt, sel_v = sel_v, n_top = 10, min_var, is_regressed = TRUE),
merge_legend = TRUE )
cat('\n\n')
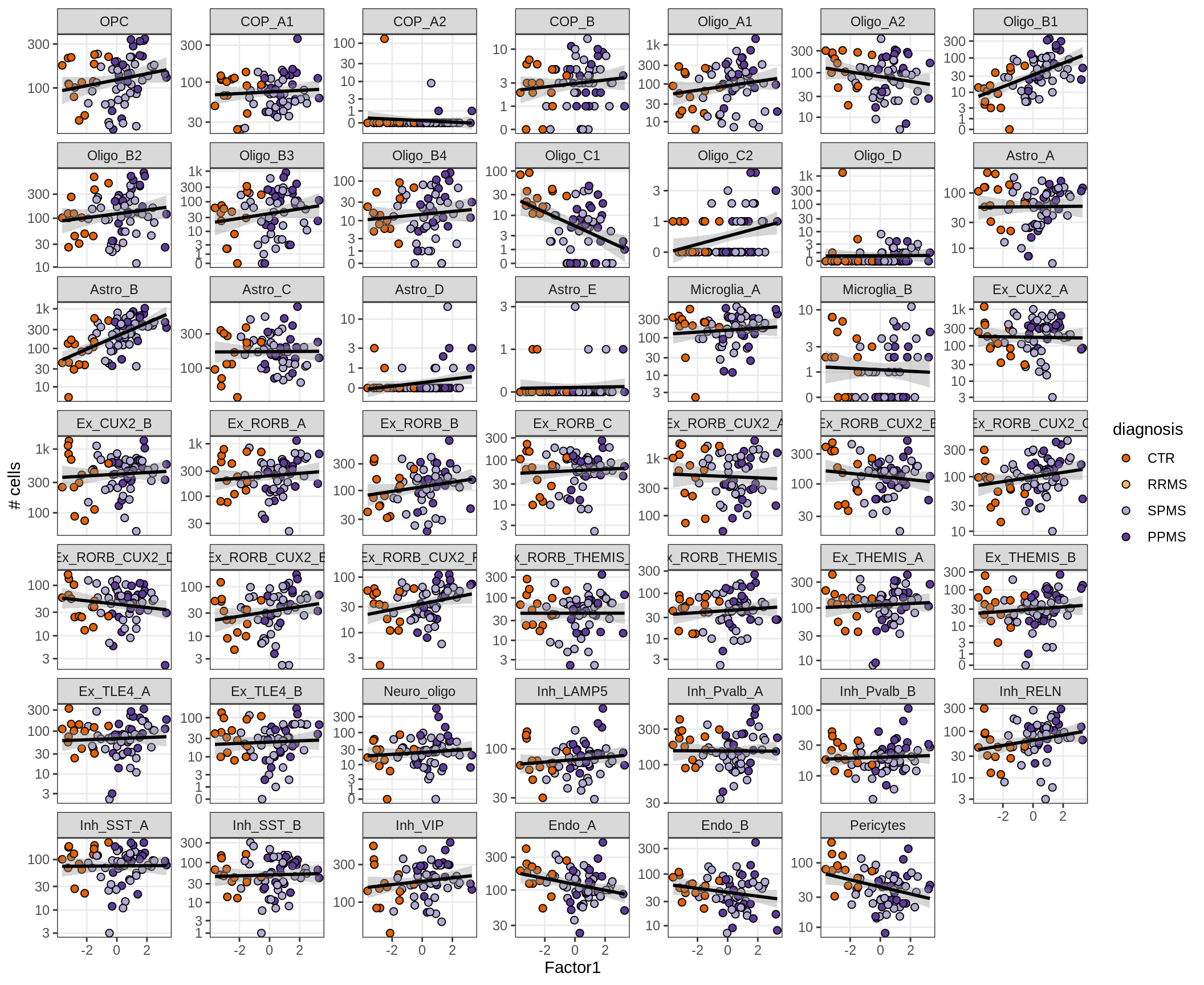
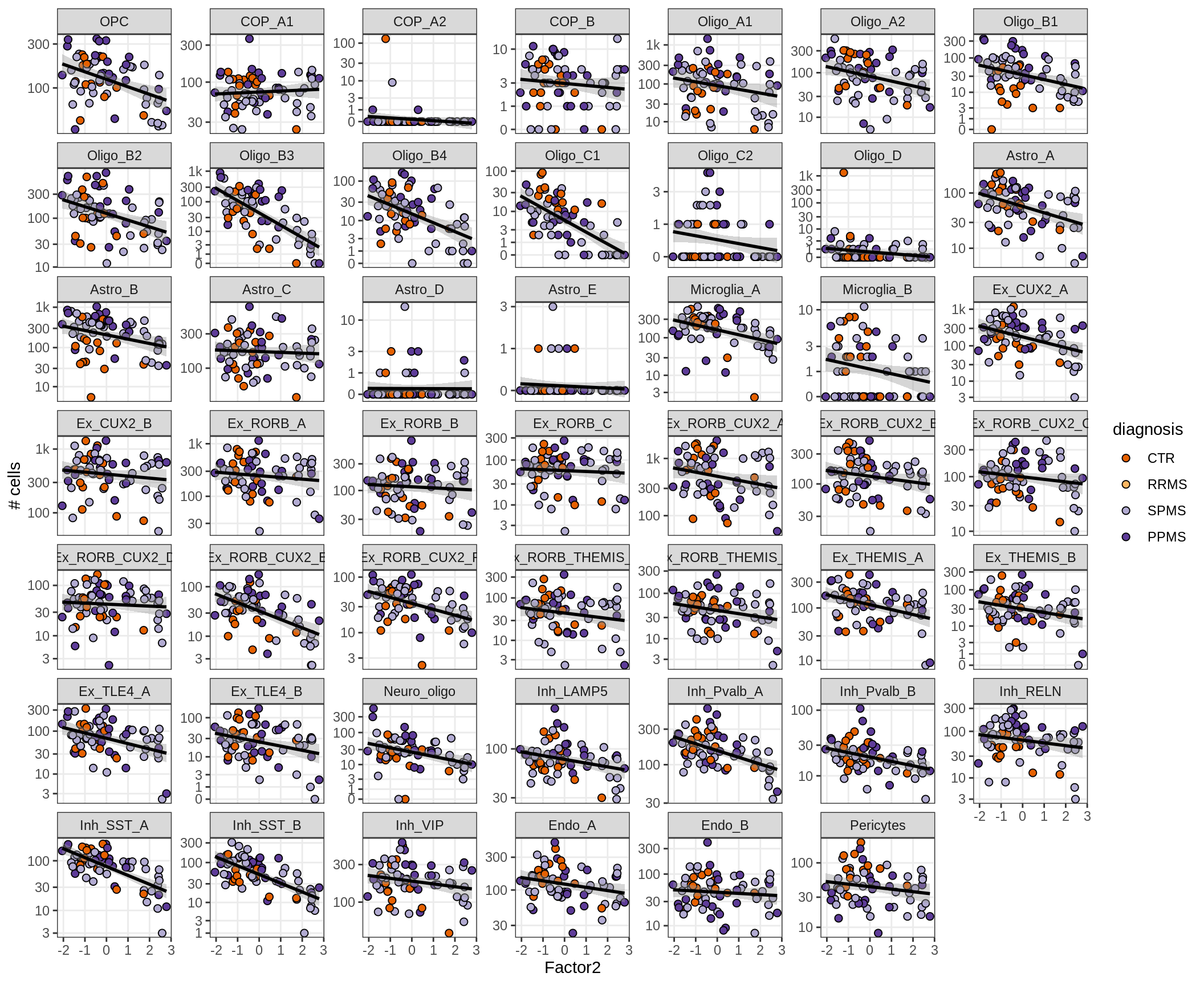
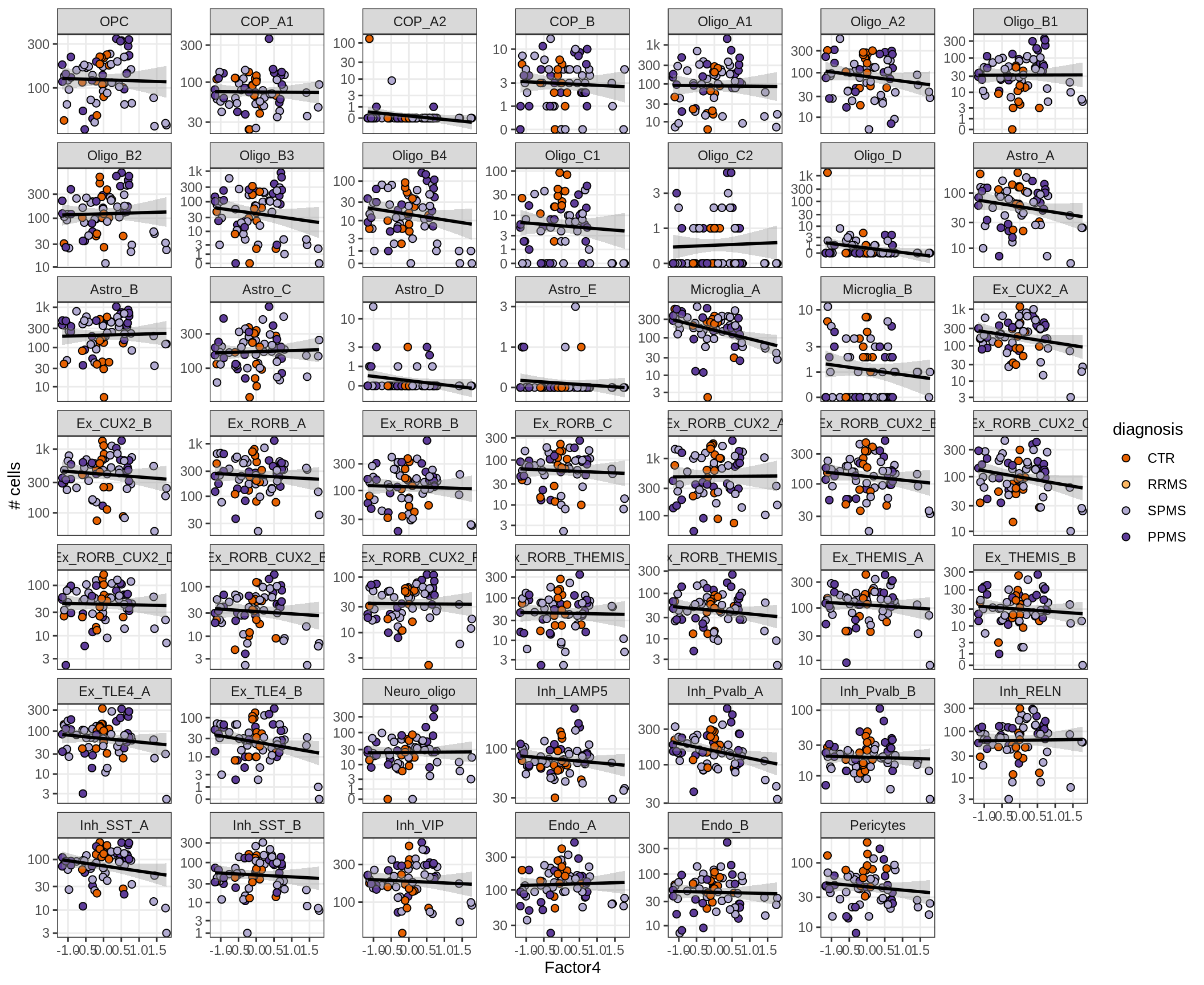
}Factors vs number of cells
for (f in factors_names(model) ) {
cat('### ', f, '\n', sep = '')
print(plot_mofa_vs_n_cells(model, n_cells_dt, sel_f = f))
cat('\n\n')
}Factors vs top genes
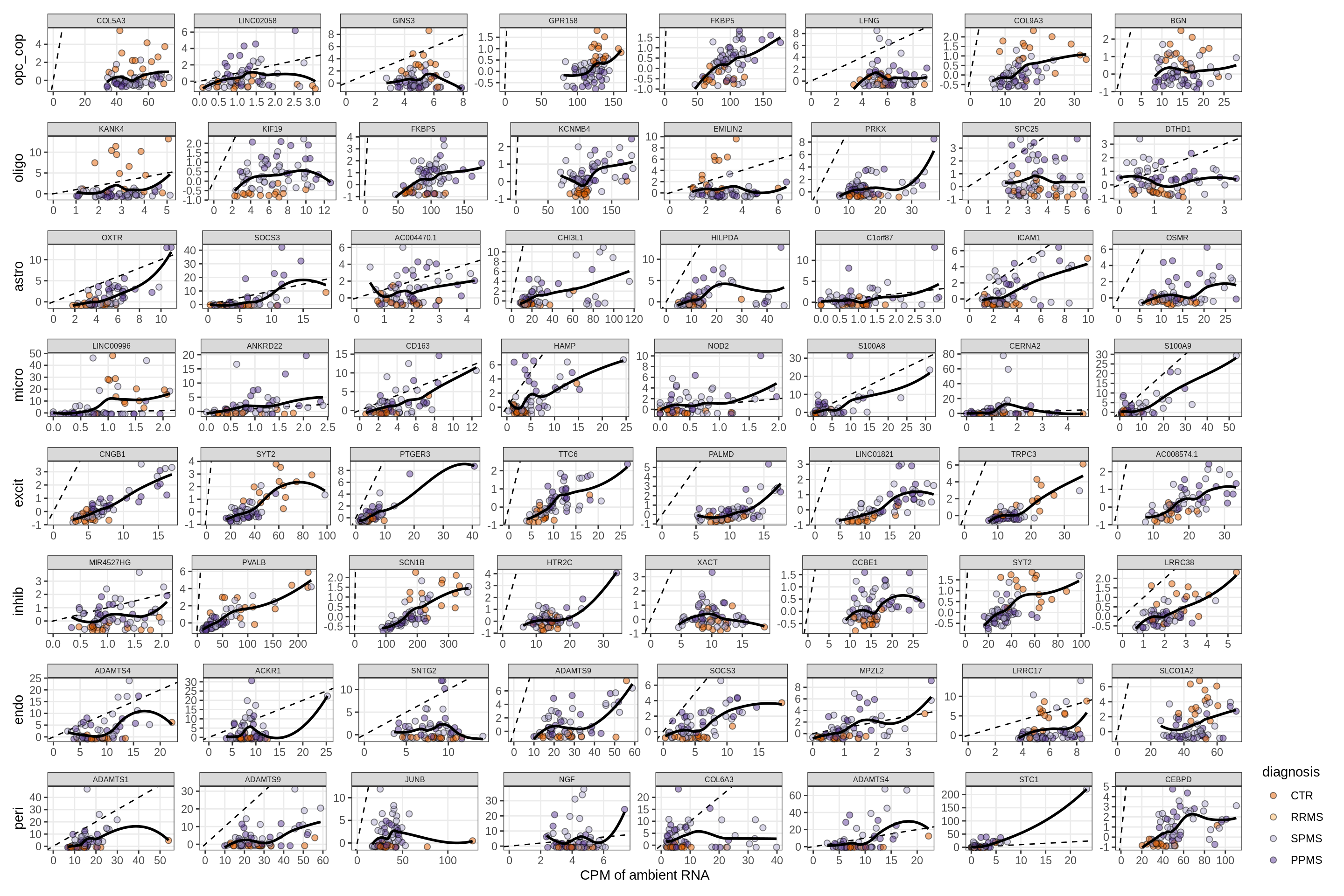
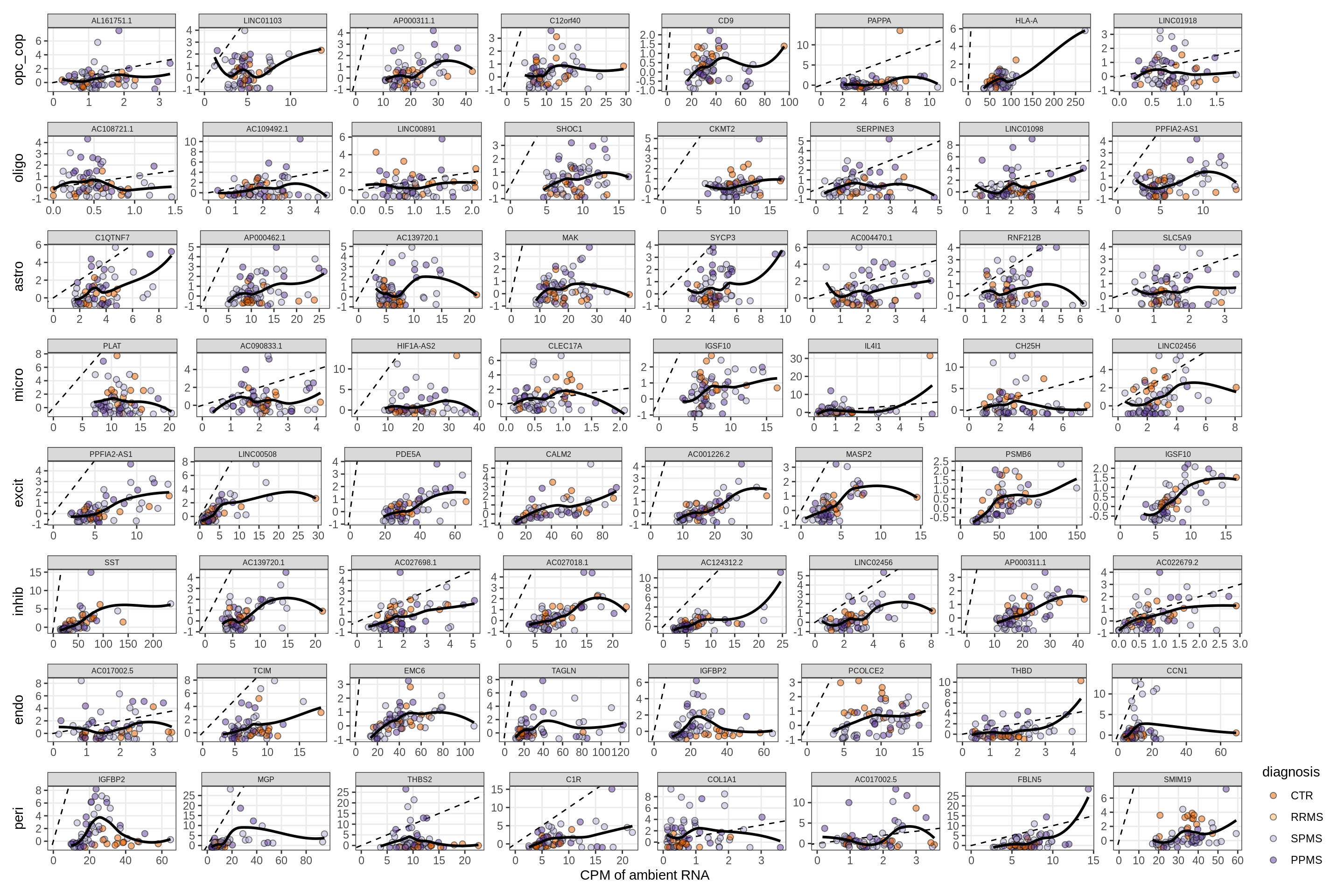
for (f in factors_names(model)) {
cat('### ', f, '\n', sep = '')
print(plot_mofa_vs_logcpm(model, annots_dt, sel_f = f))
cat('\n\n')
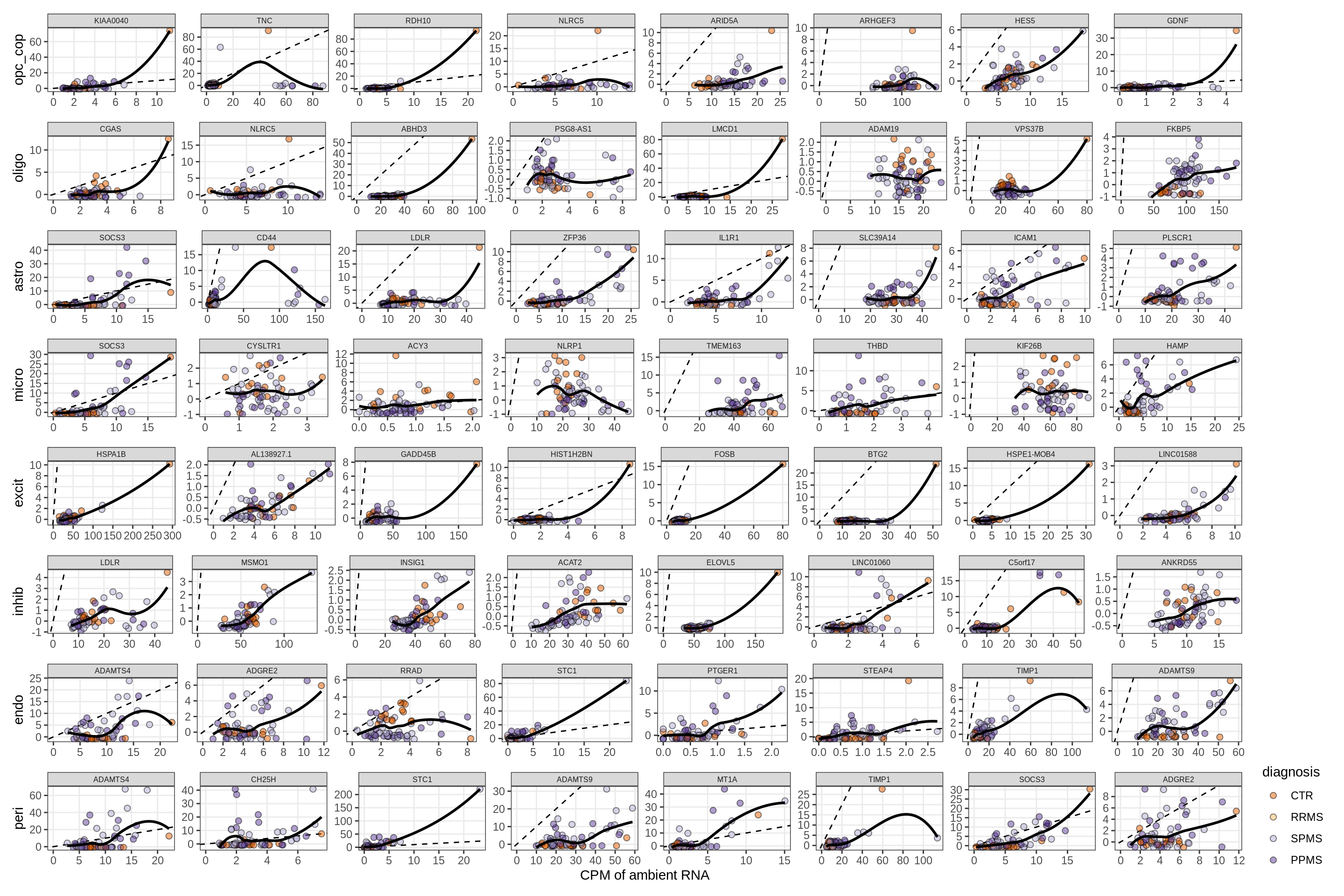
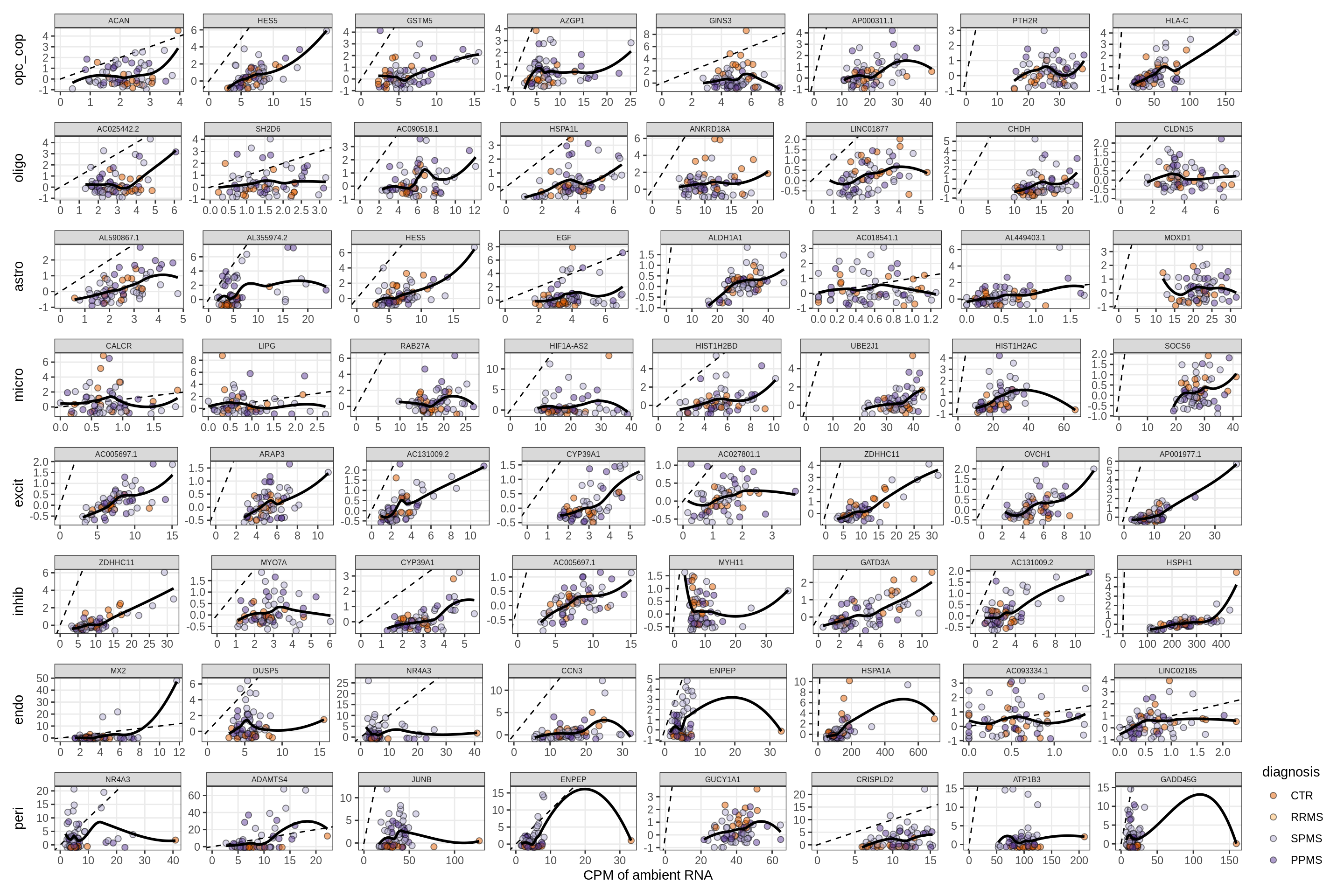
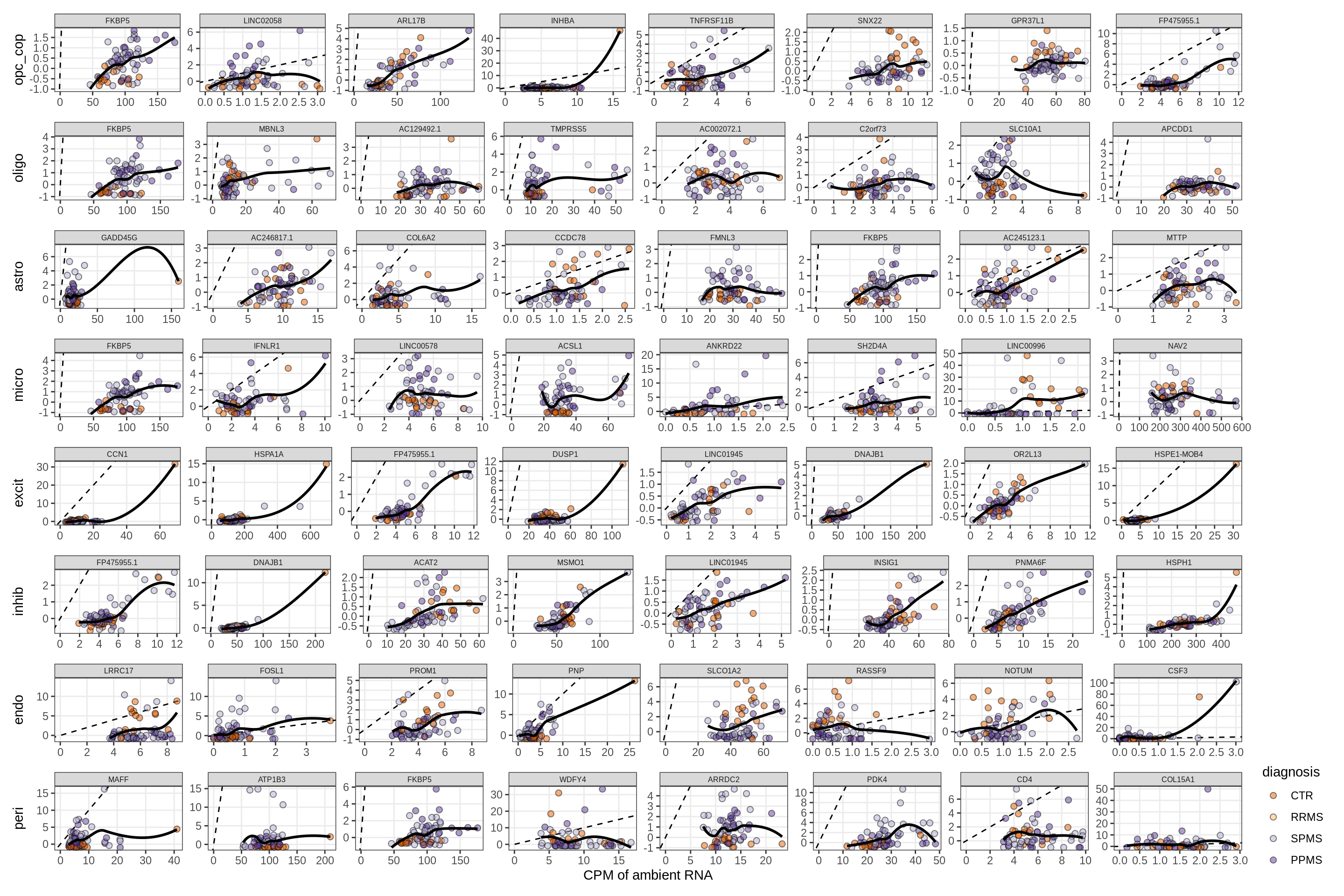
}Factors vs top genes - soup
for (f in factors_names(model) ) {
cat('### ', f, '\n', sep = '')
print(plot_mofa_vs_soup_logcpm(model, annots_dt, soup_dt,
sel_f = f, trans = 'linear'))
cat('\n\n')
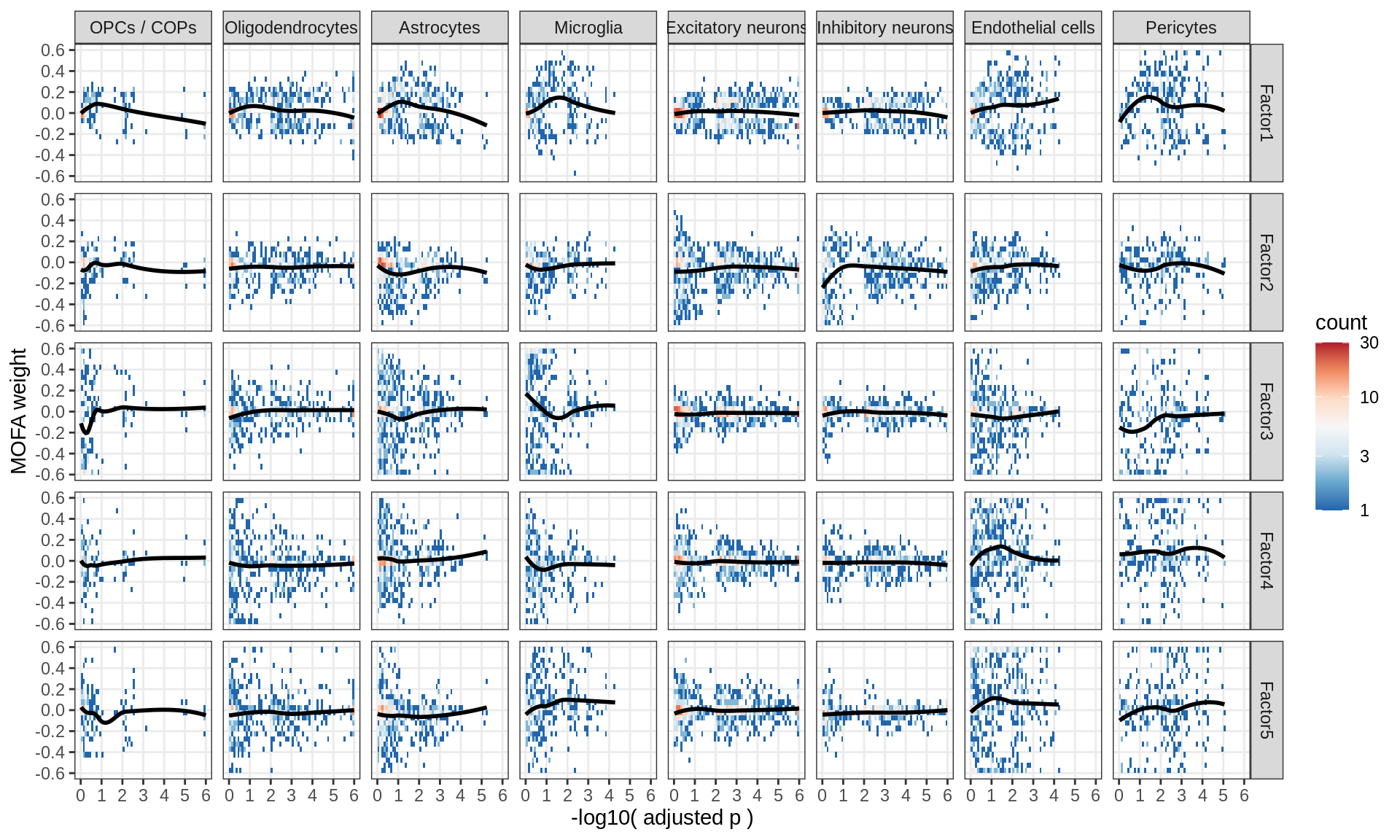
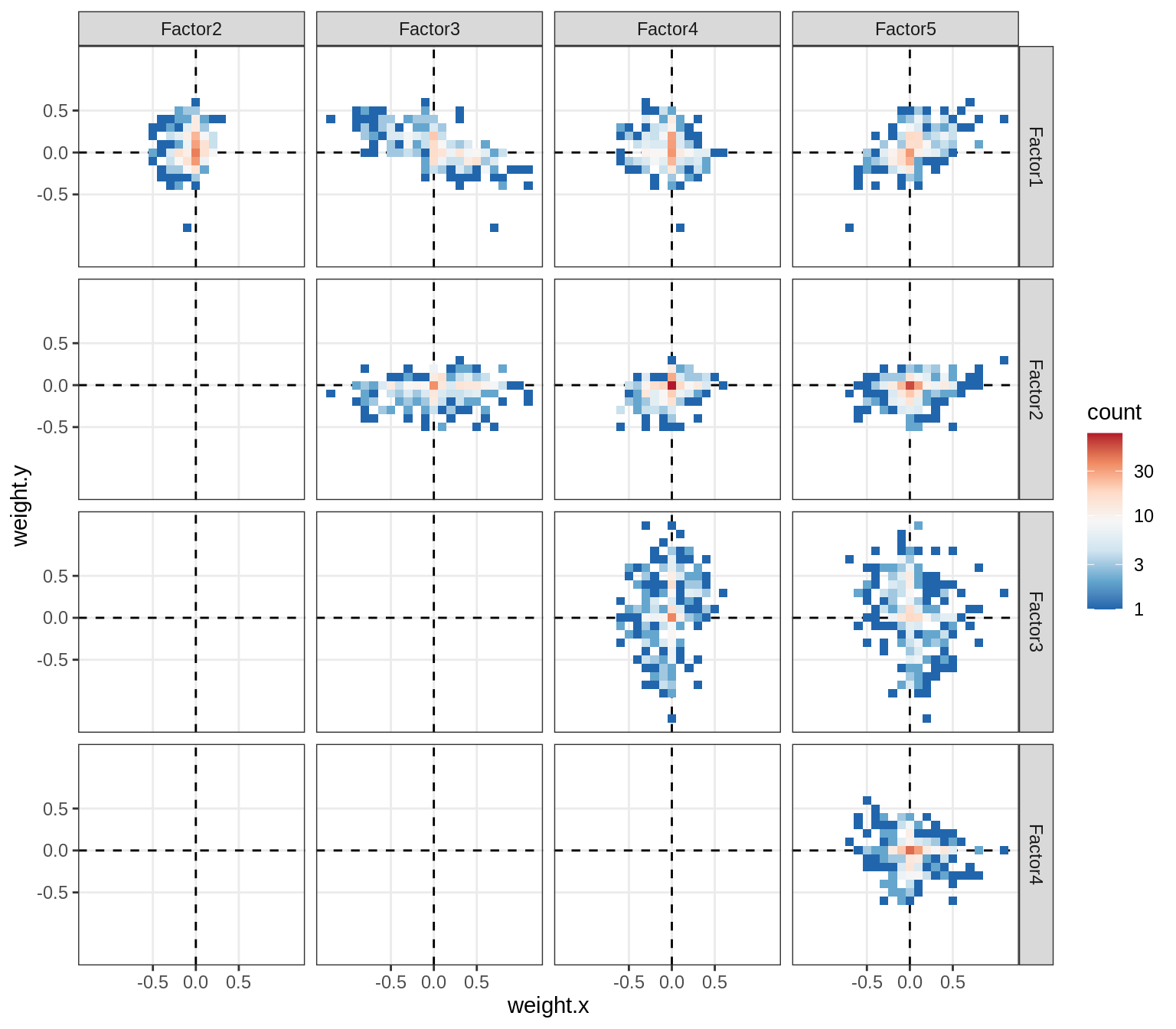
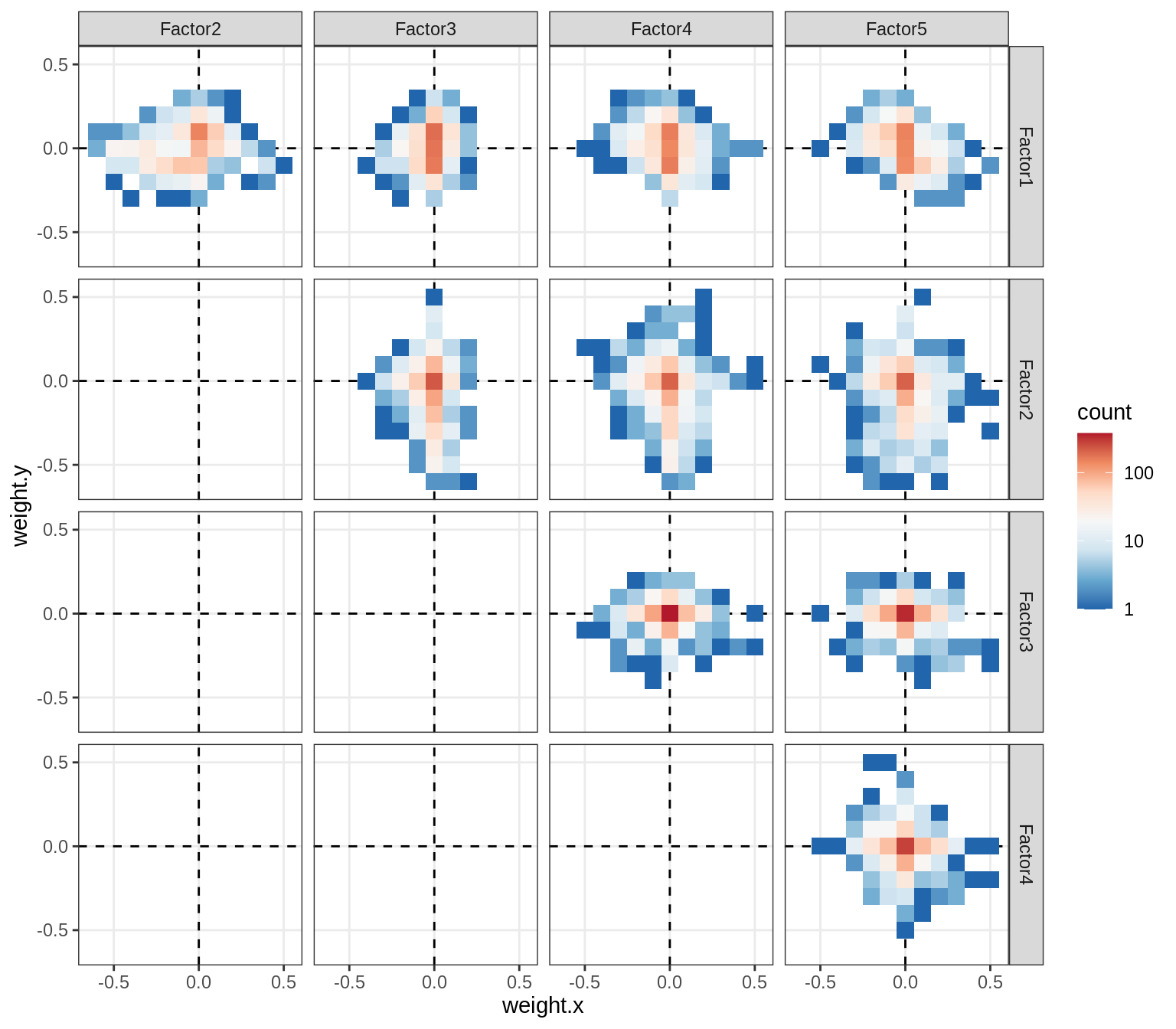
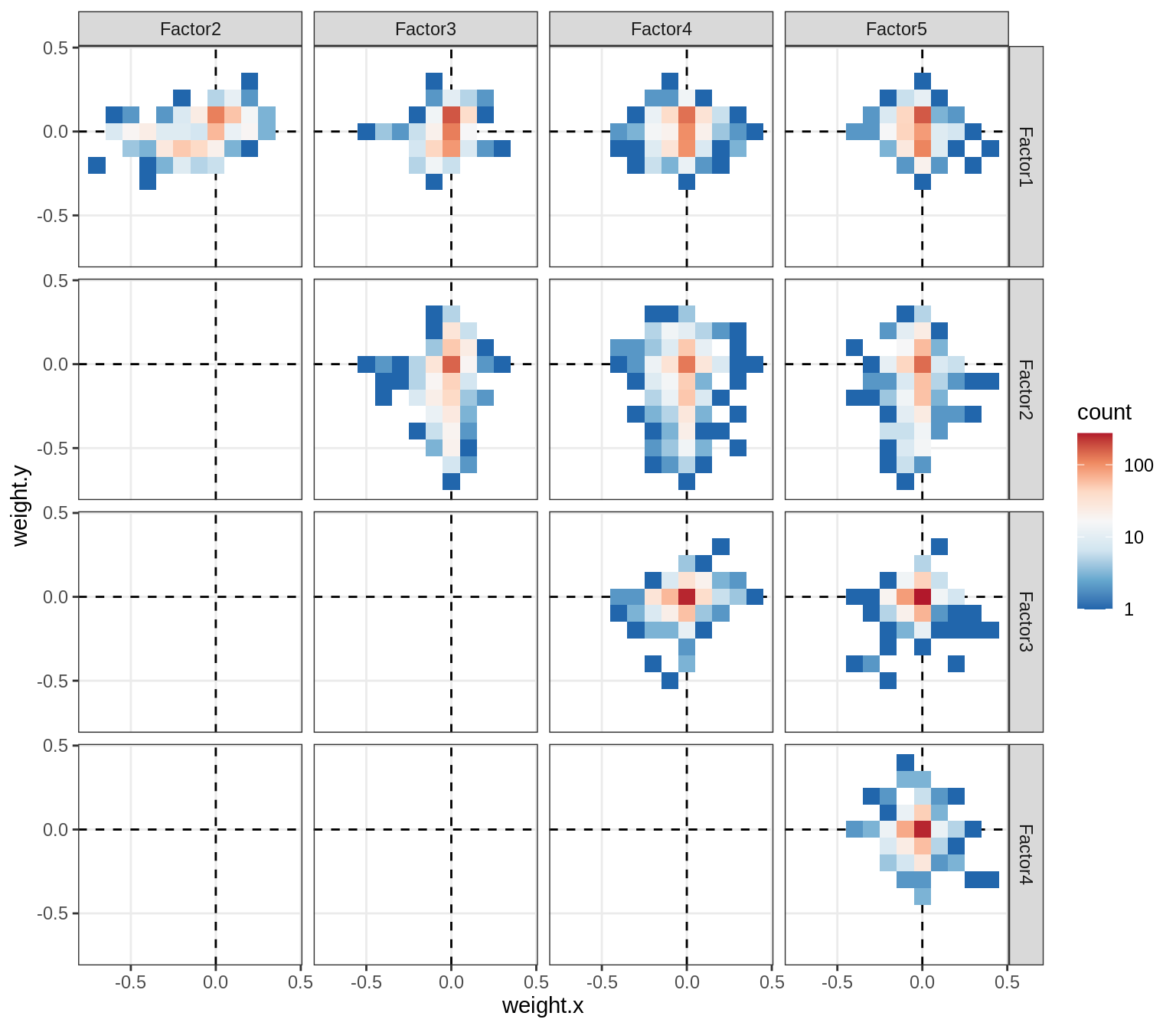
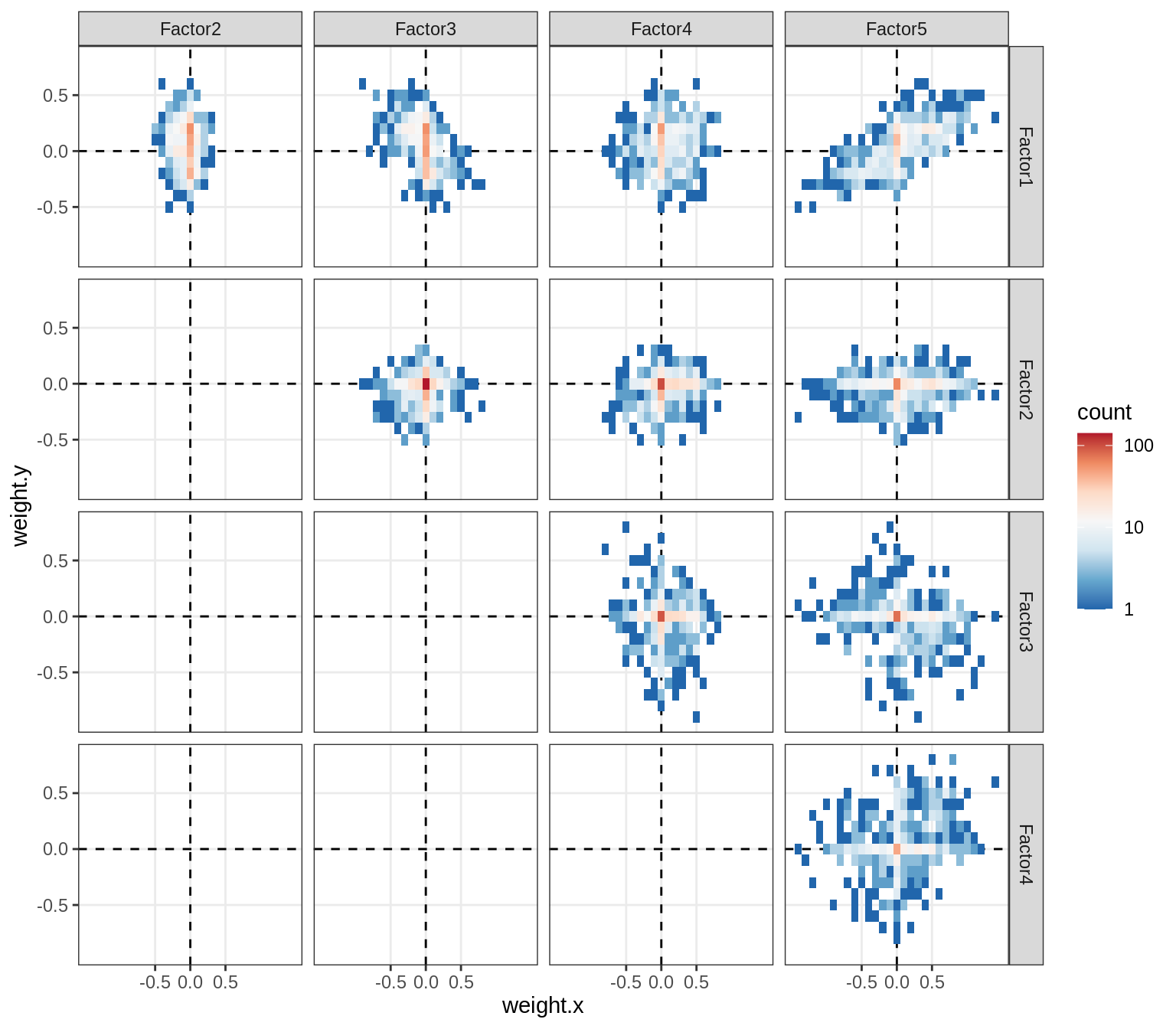
}Distributions of factor weights
(plot_mofa_weights(model))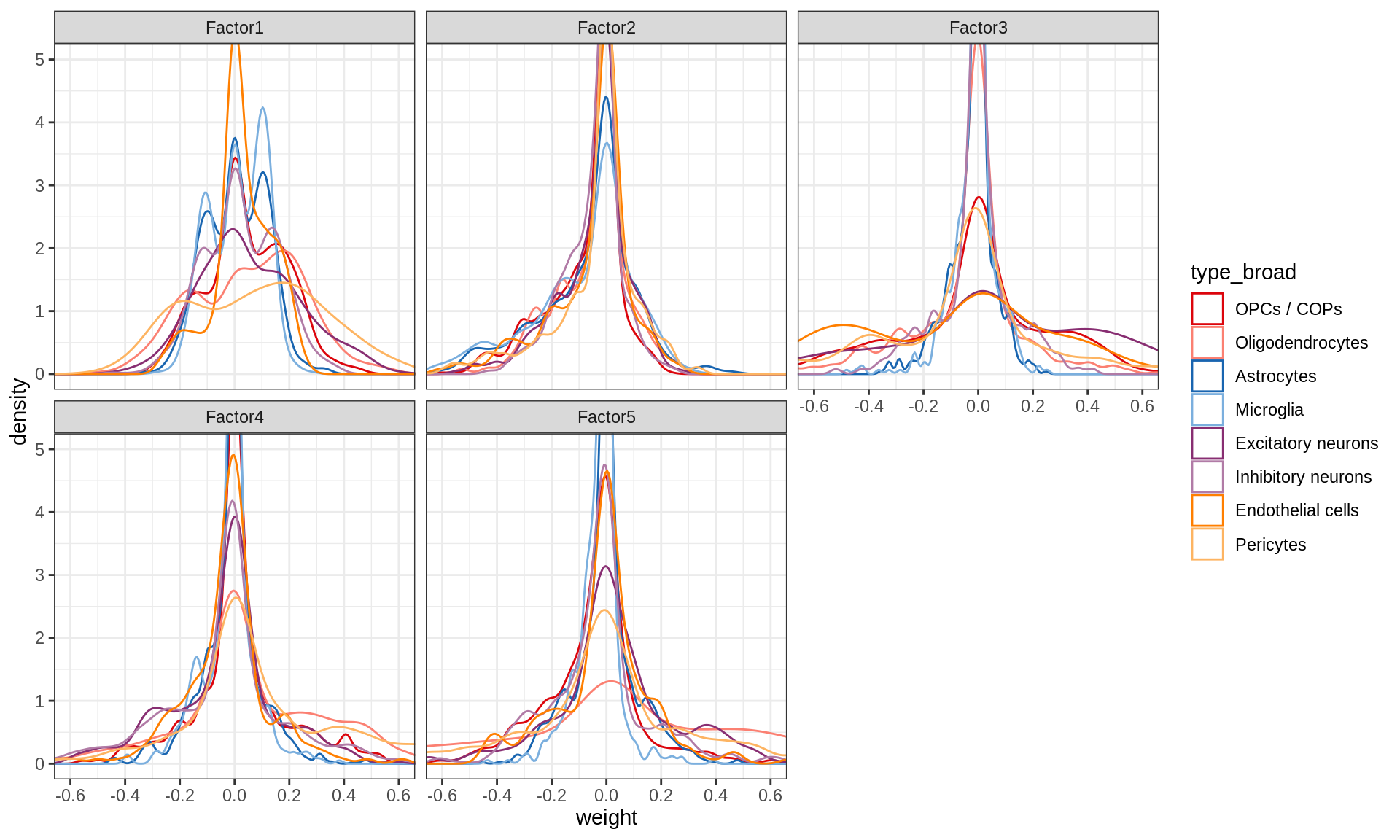
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
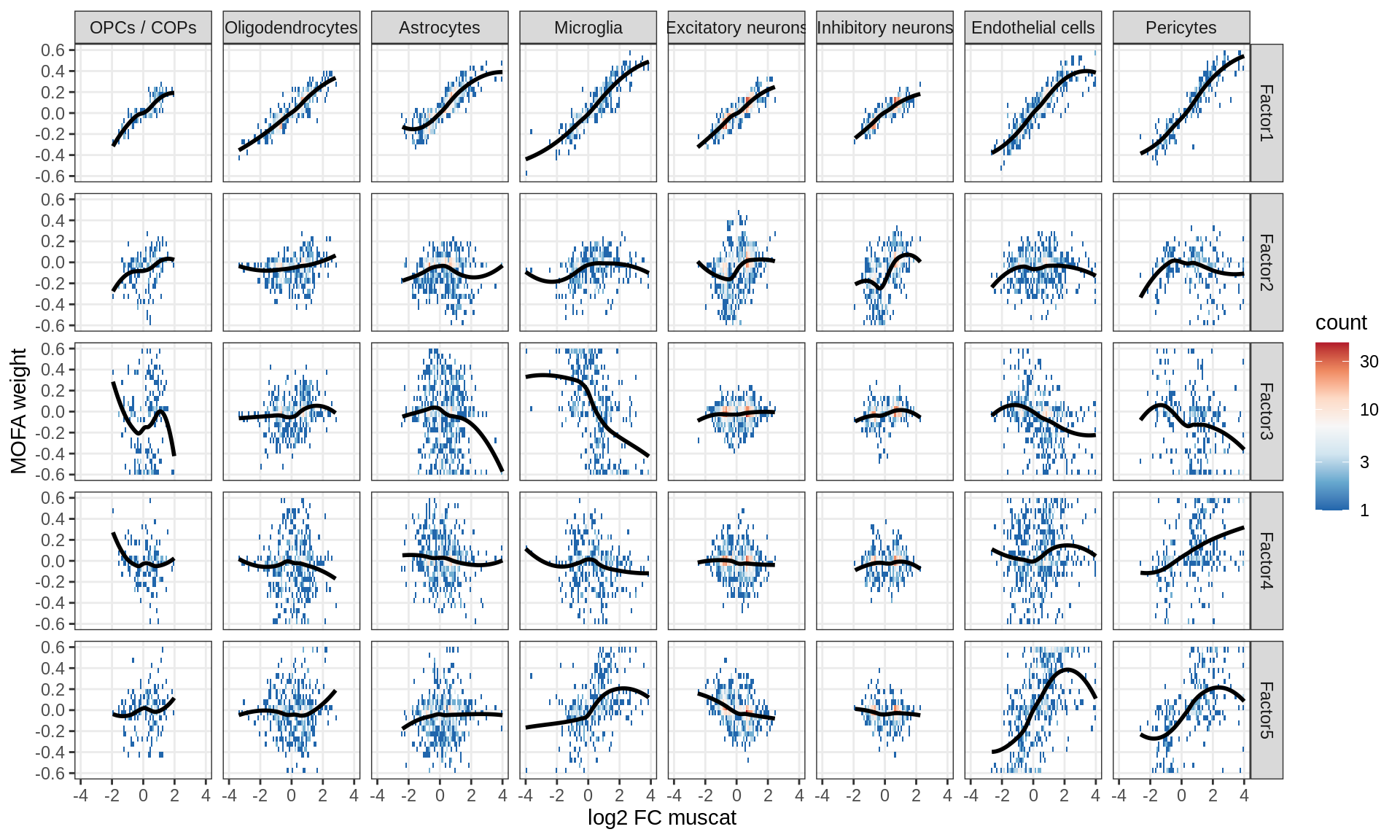
Factor weights vs muscat results
for (what in c('log10_padj', 'log2FC')) {
cat('### ', what, '\n', sep = '')
print(plot_muscat_vs_mofa(model, filter_dt, what = what))
cat('\n\n')
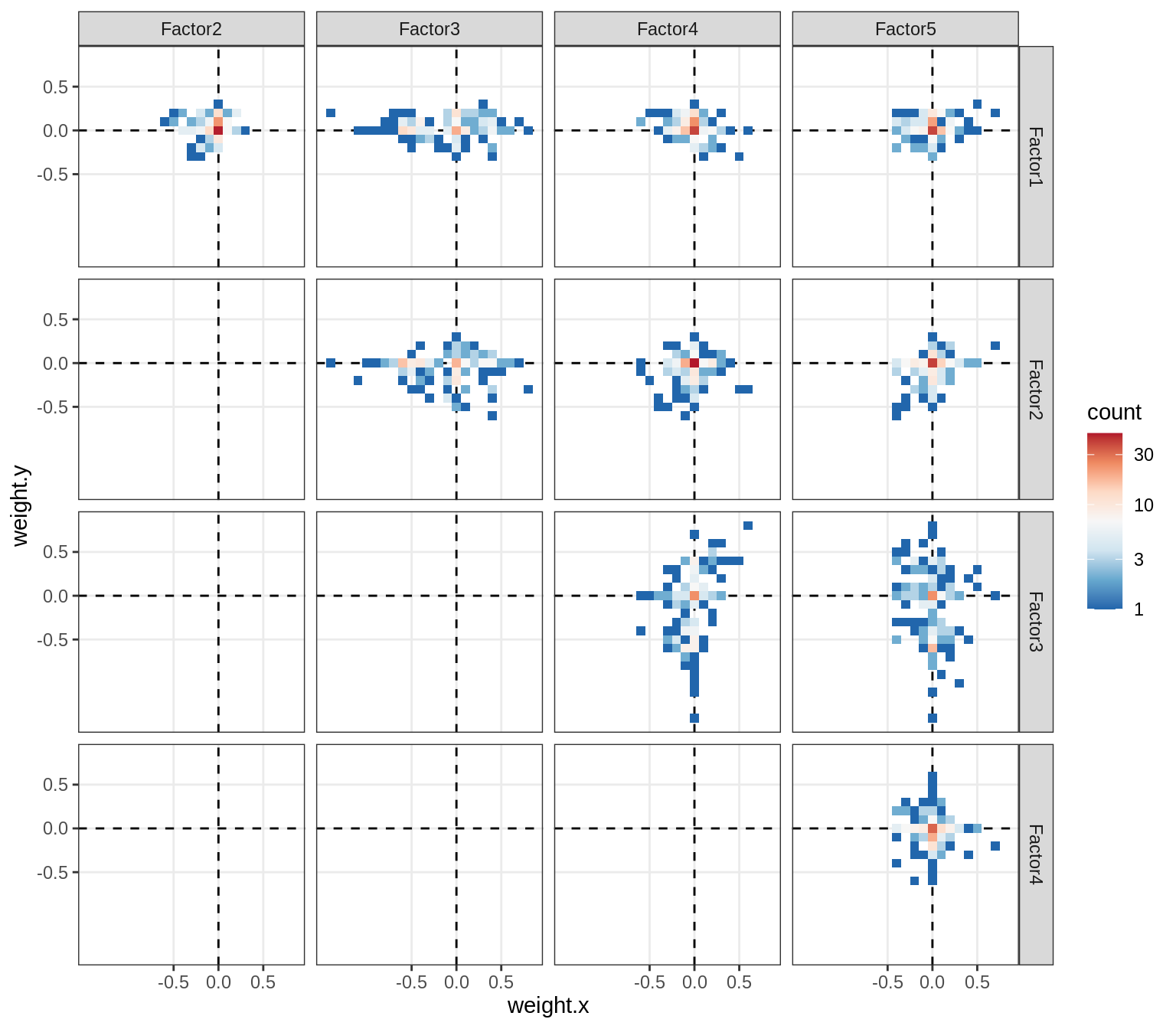
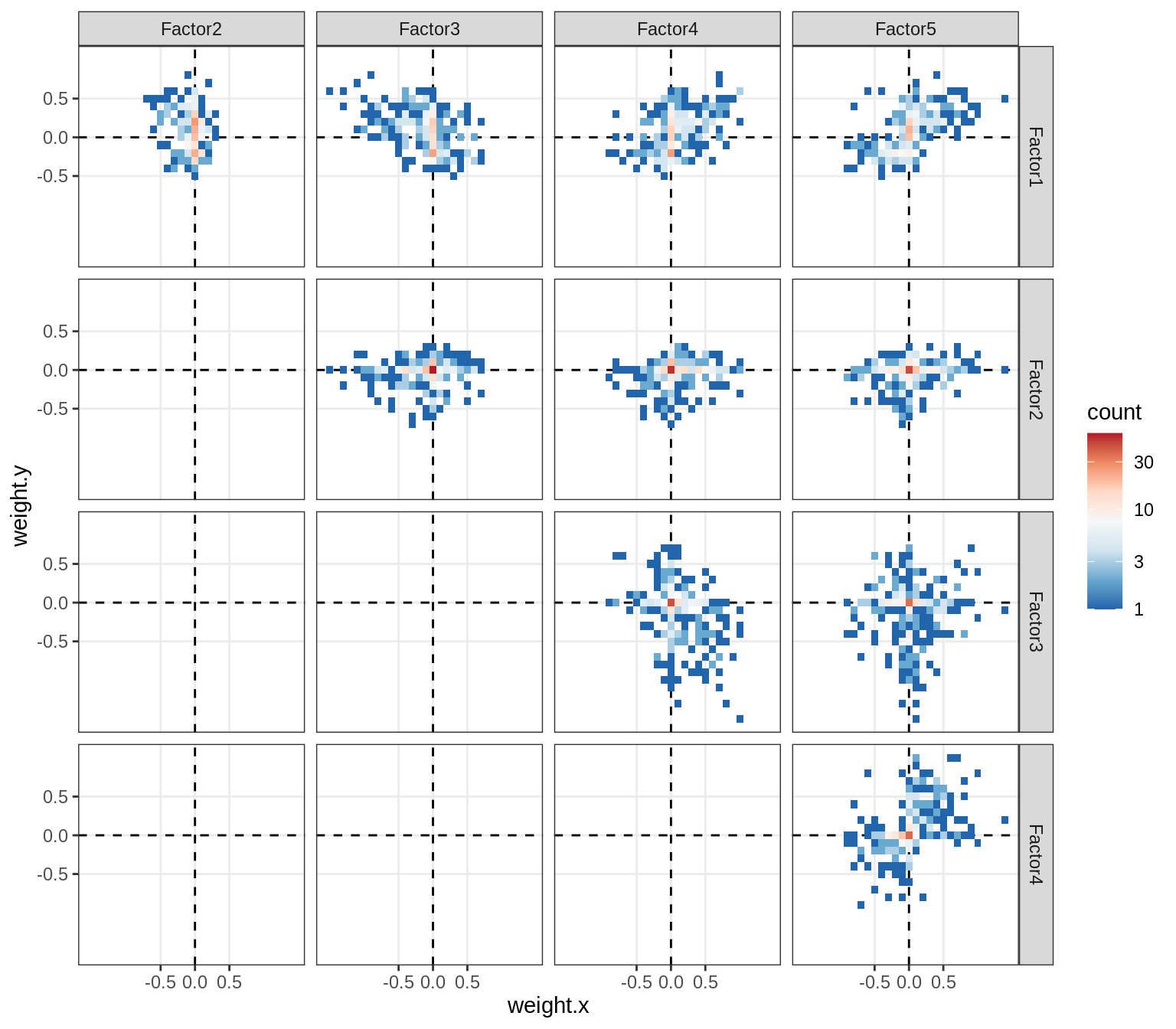
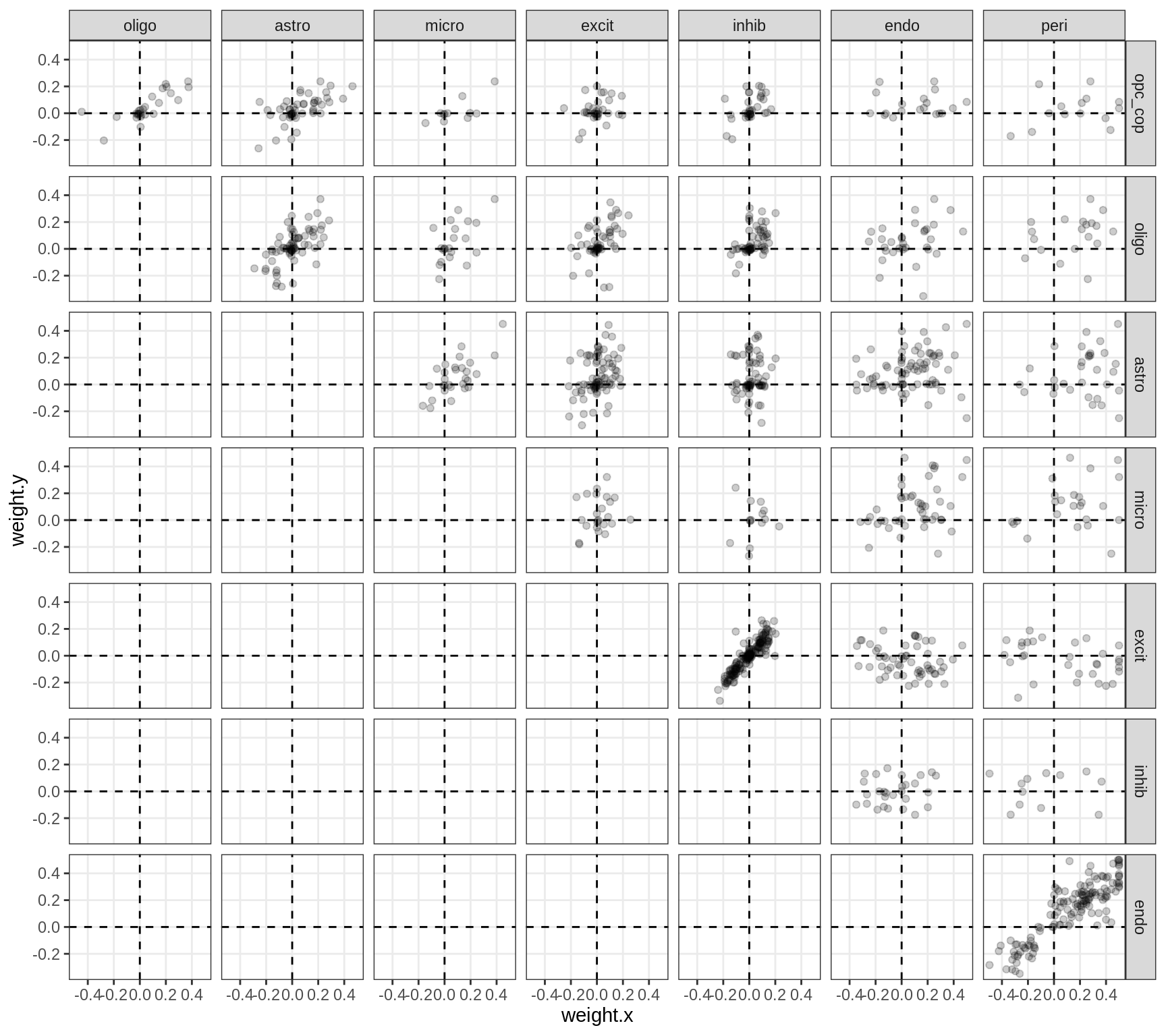
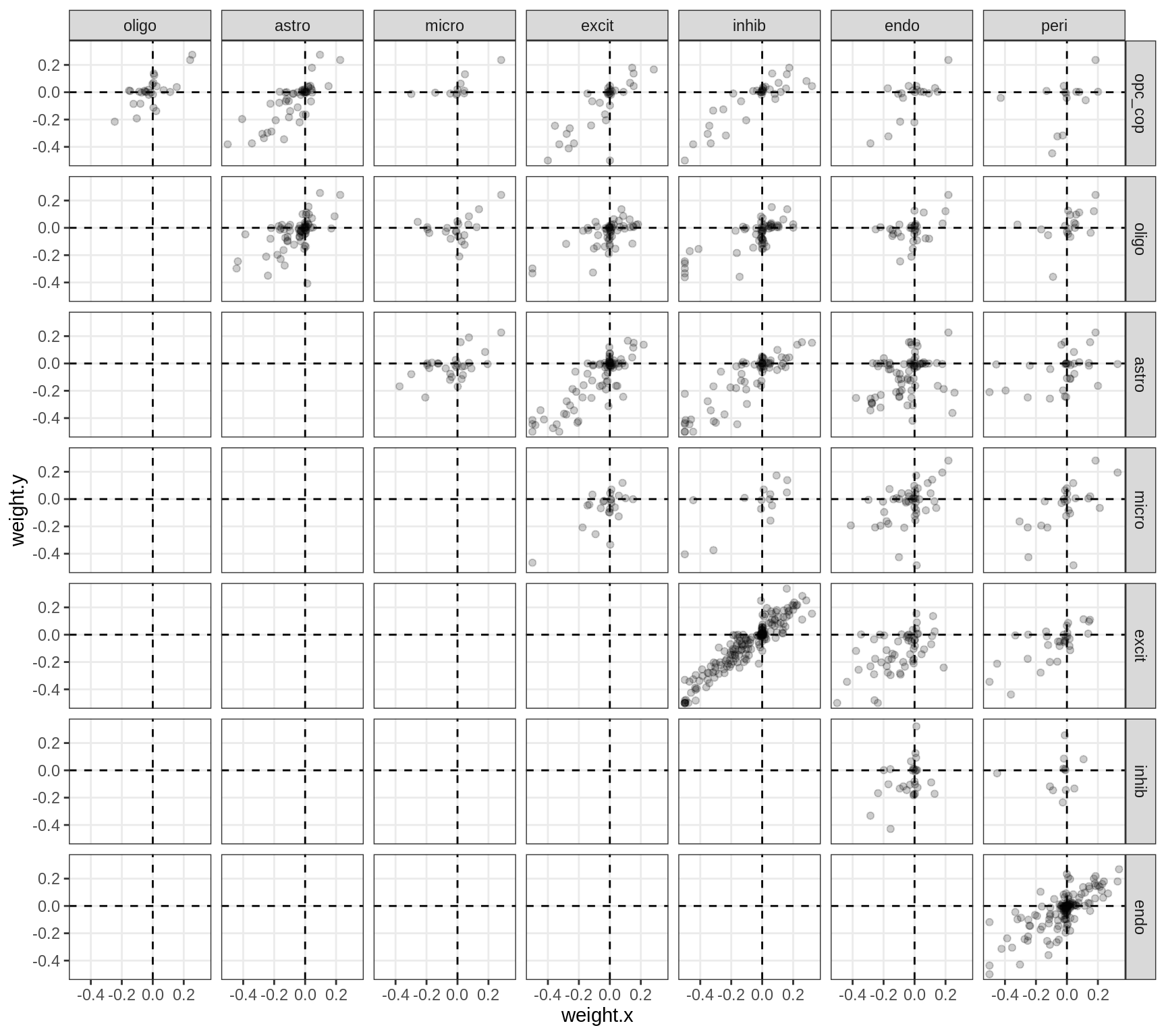
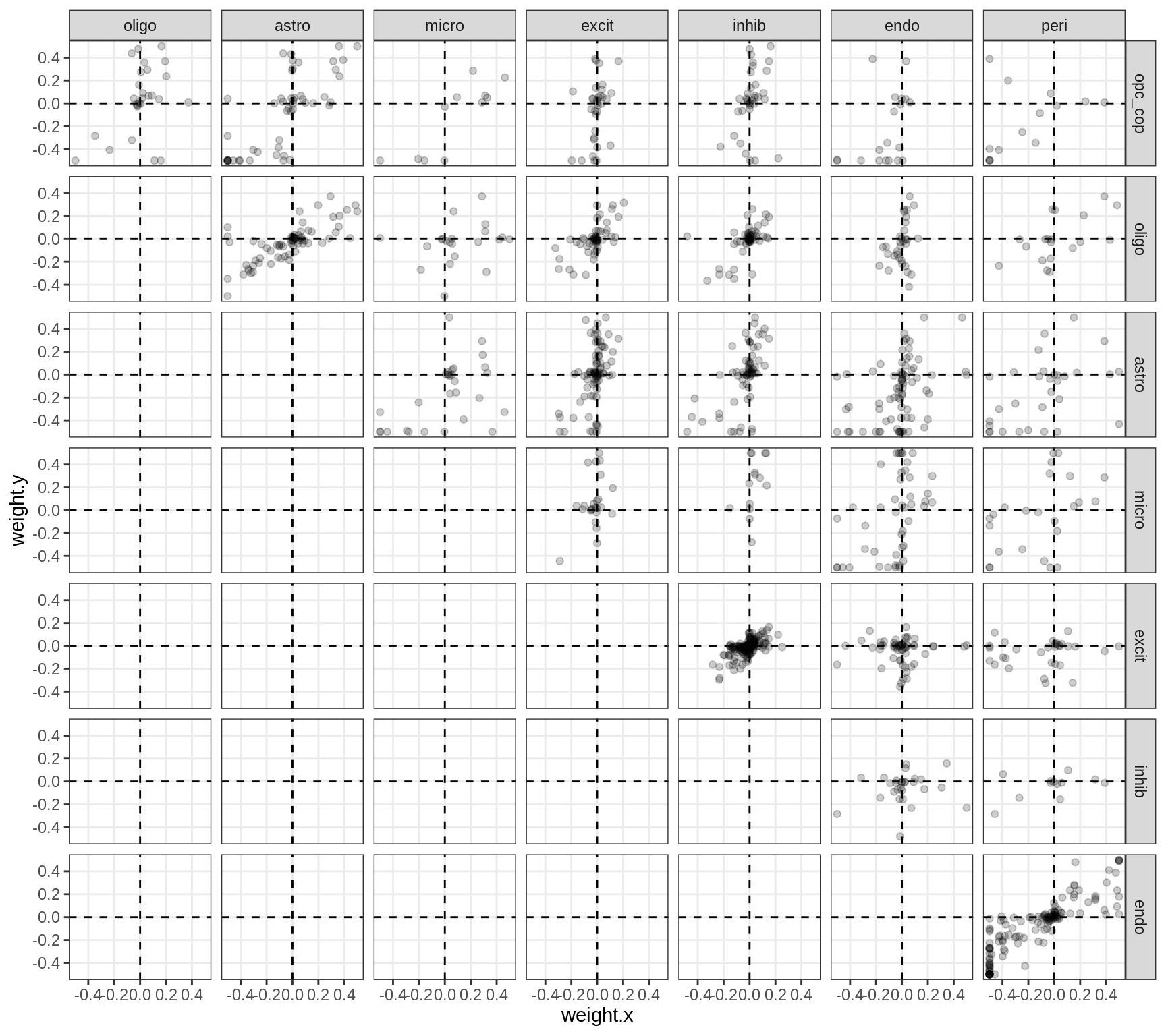
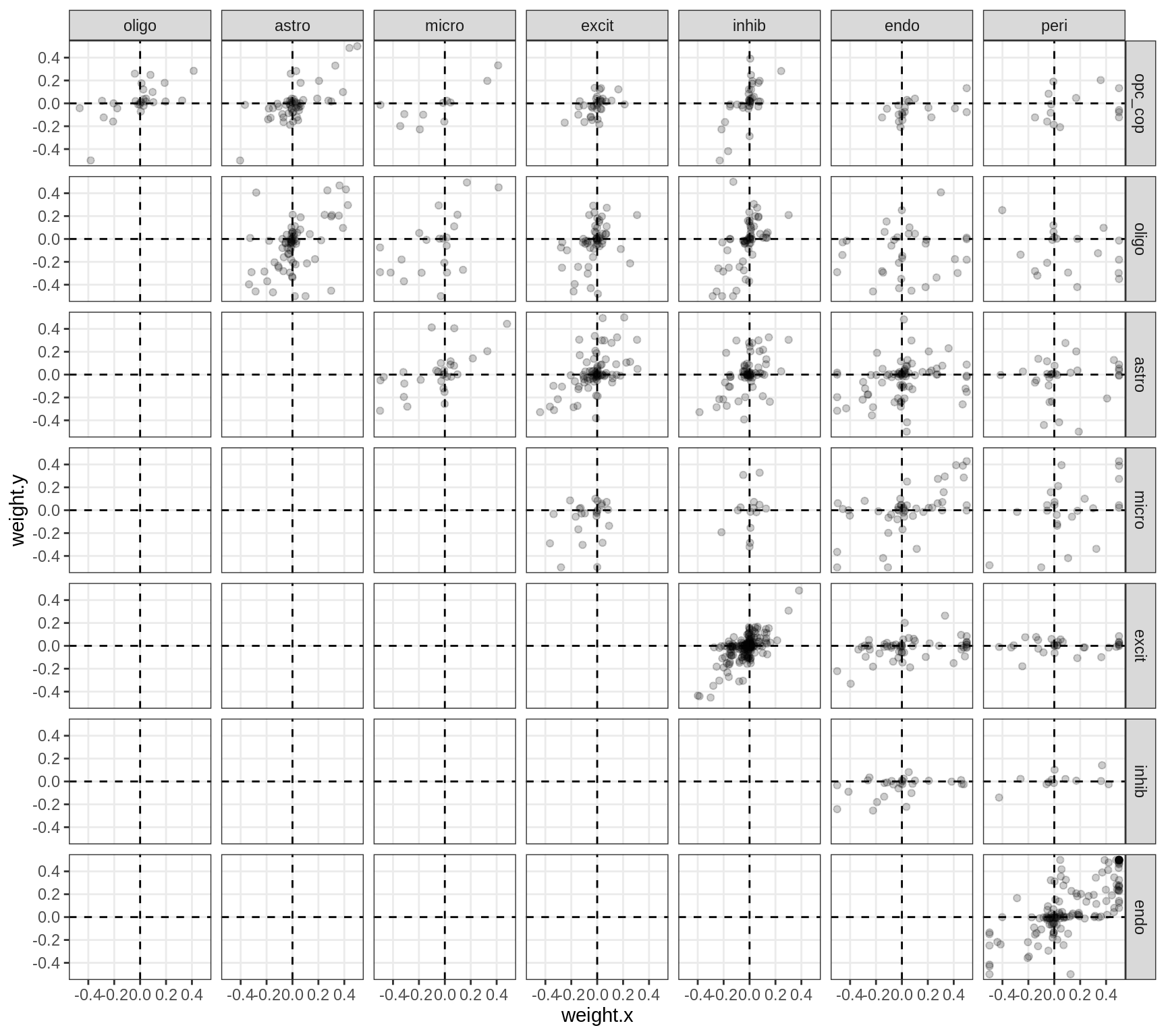
}Correlations between factor weights - split by celltype
for (v in broad_short[sel_cl]) {
cat('### ', v, '\n', sep = '')
print(plot_factor_weight_corrs(model, v, by = 'type', how = 'bin'))
cat('\n\n')
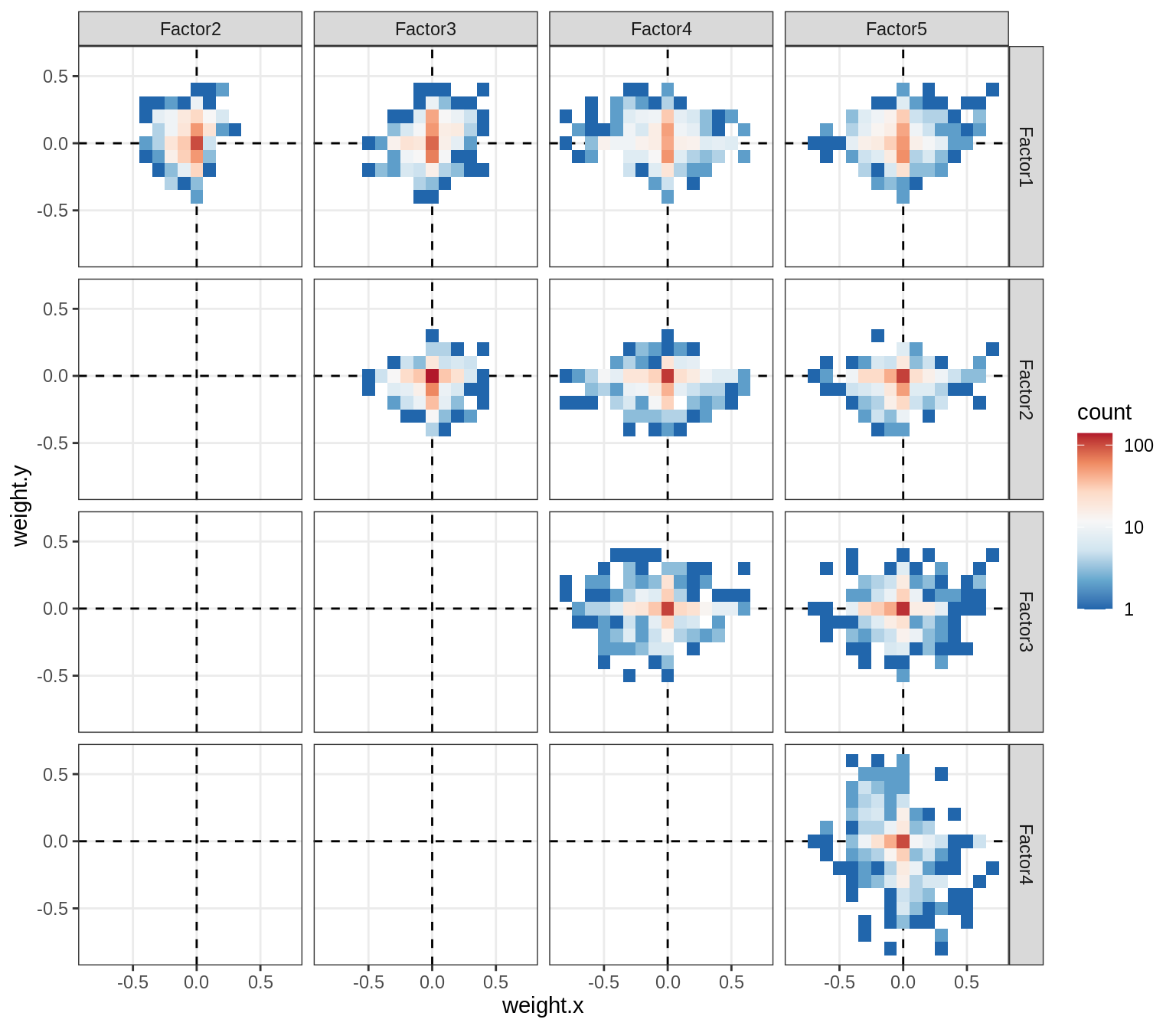
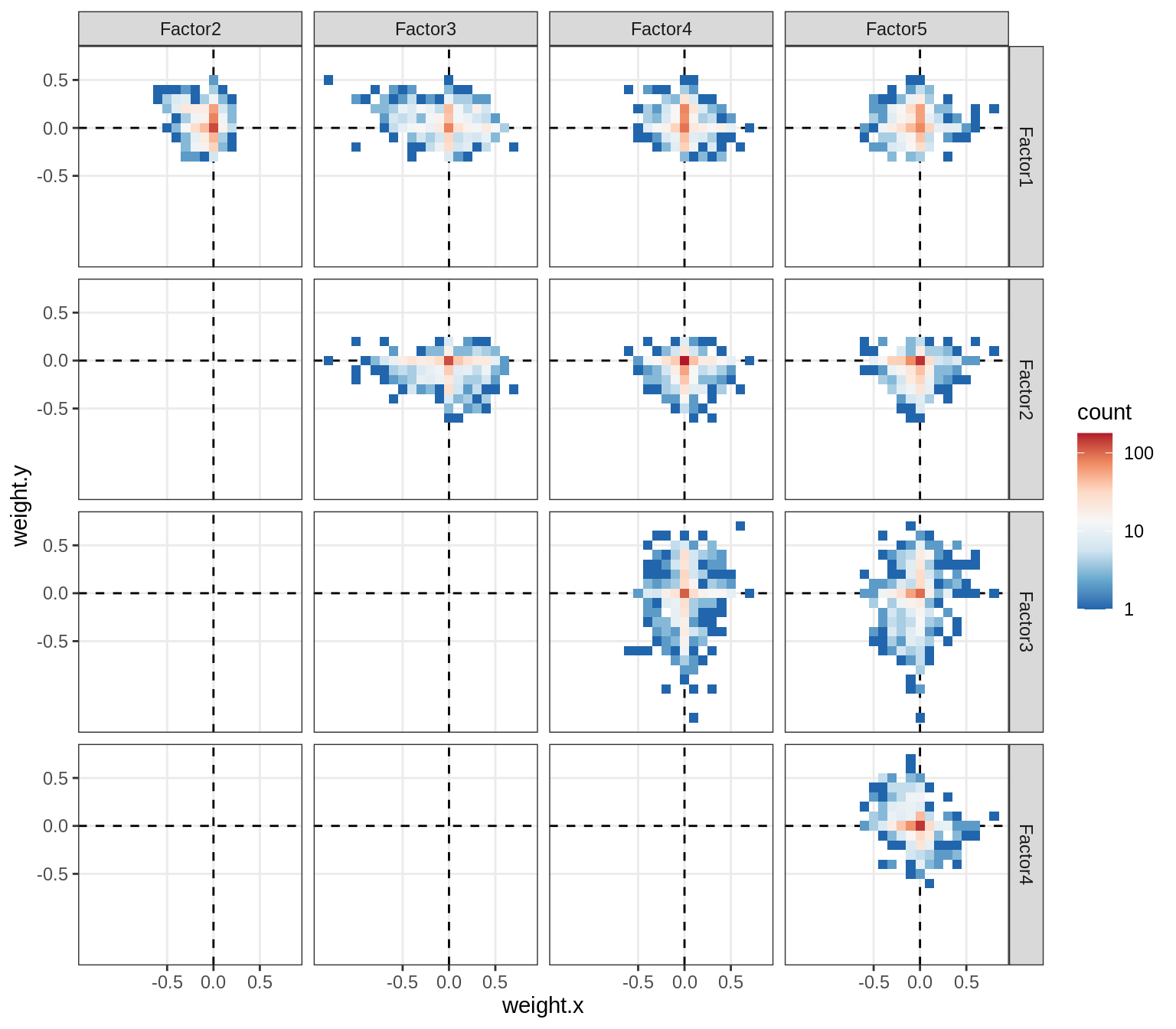
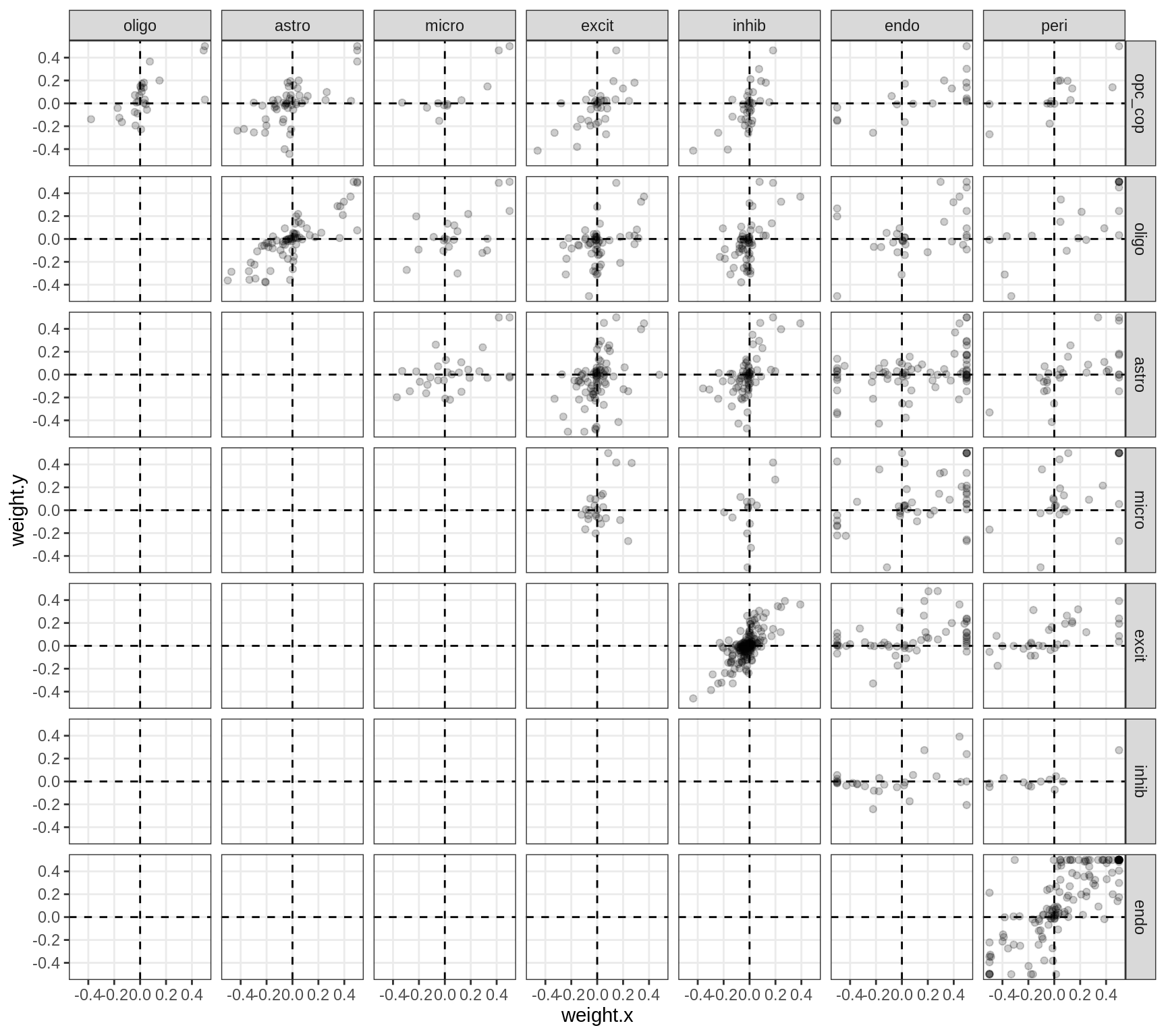
}Correlations between factor weights - split by factor
for (f in factors_names(model) ) {
cat('### ', f, '\n', sep = '')
print(plot_factor_weight_corrs(model, f, by = 'factor', how = 'point'))
cat('\n\n')
}GSEA for factors
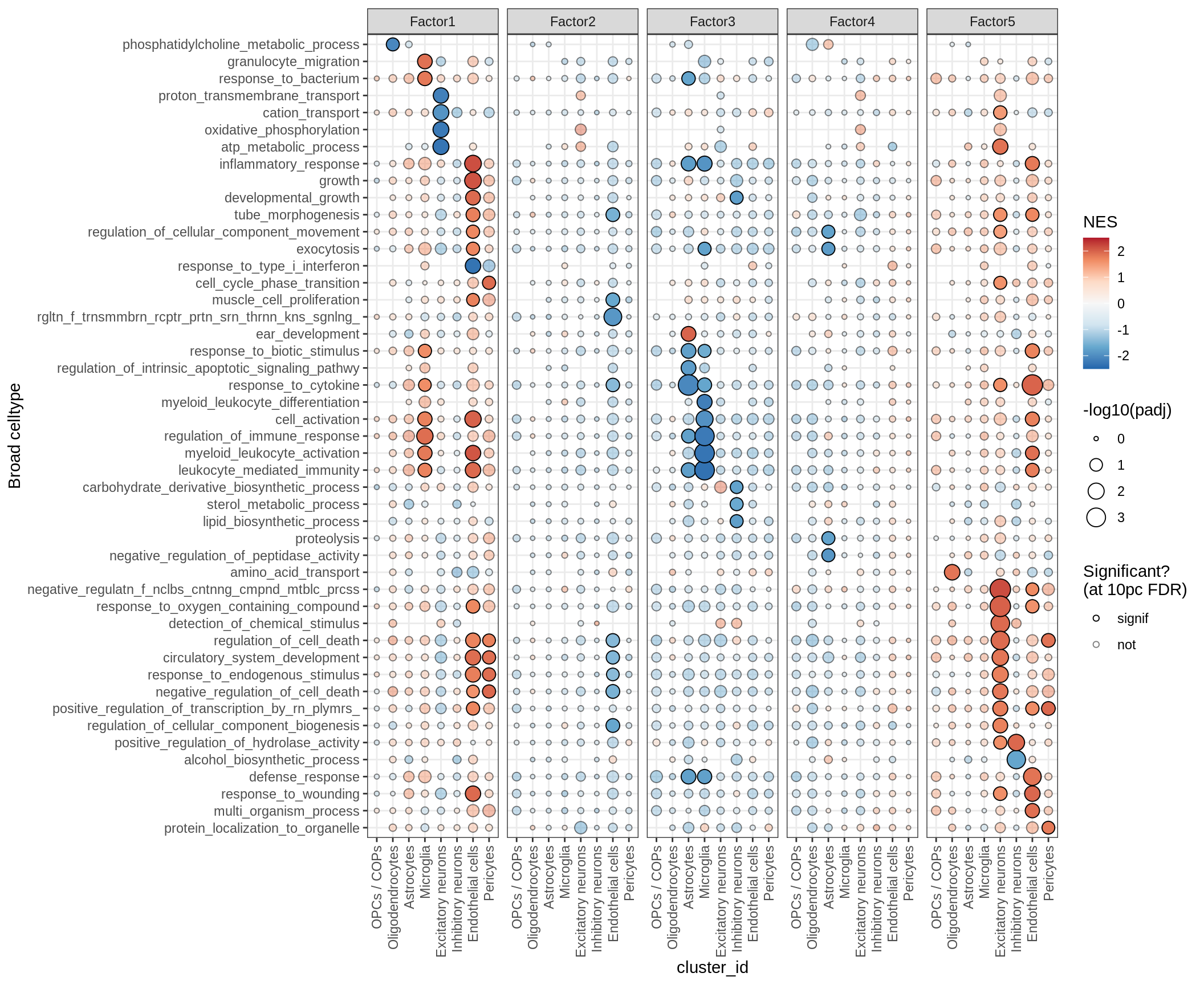
for (p in names(gsea_list)) {
# restrict to relevant GO terms
cat('### ', p, '\n', sep='')
dt = gsea_list[[p]]
if (nrow(dt[ main_path == TRUE ]) == 0)
next
# plot
print(plot_mofa_gsea_dotplot(dt, labels_dt,
fgsea_cut = fgsea_cut, n_total = 60))
cat('\n\n')
}Outputs
Top filter genes
# merge filtered and weights
xls_dt = calc_xls_dt(model, filtered_dt)
# save outputs
write_xlsx(list(mofa_weights = xls_dt), path = interesting_f)Figures
Illustrative example
for (g in example_gs) {
cat('### ', str_extract(g, '^[^_]+'), '\n', sep = '')
suppressWarnings(print(plot_ranef_example(pb, example_cl, g)))
cat('\n\n')
}muscat results vs SD
(plot_muscat_vs_sd_min(res_dt, sd_dt, sel_cl, min_sd, max_p))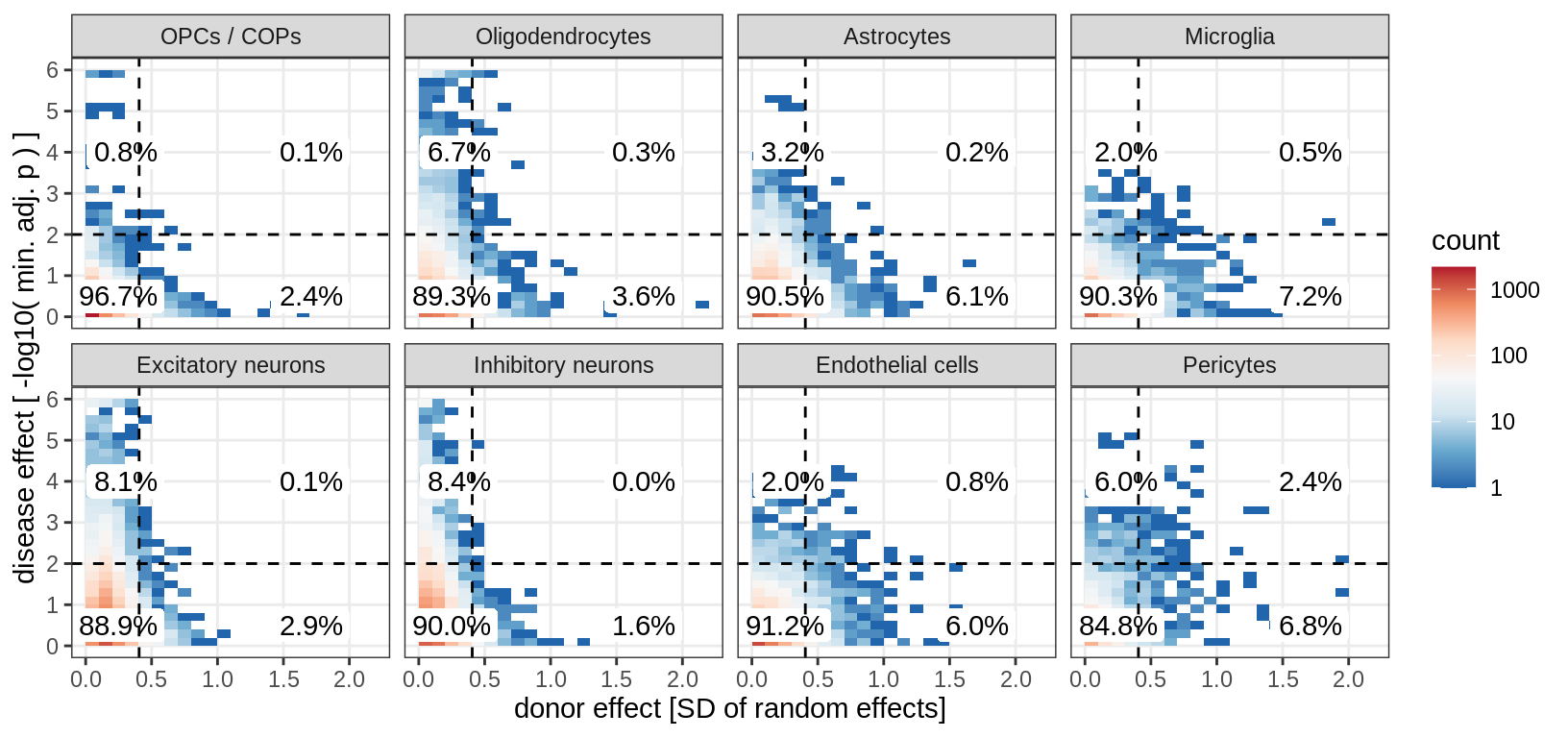
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
muscat results vs SD, MAGMA hits only
magma_hits = magma_dt[ p_magma_adj < 0.1 ]$gene_id
(plot_muscat_vs_sd_min(res_dt[ gene_id %in% magma_hits ], sd_dt,
sel_cl, min_sd, max_p))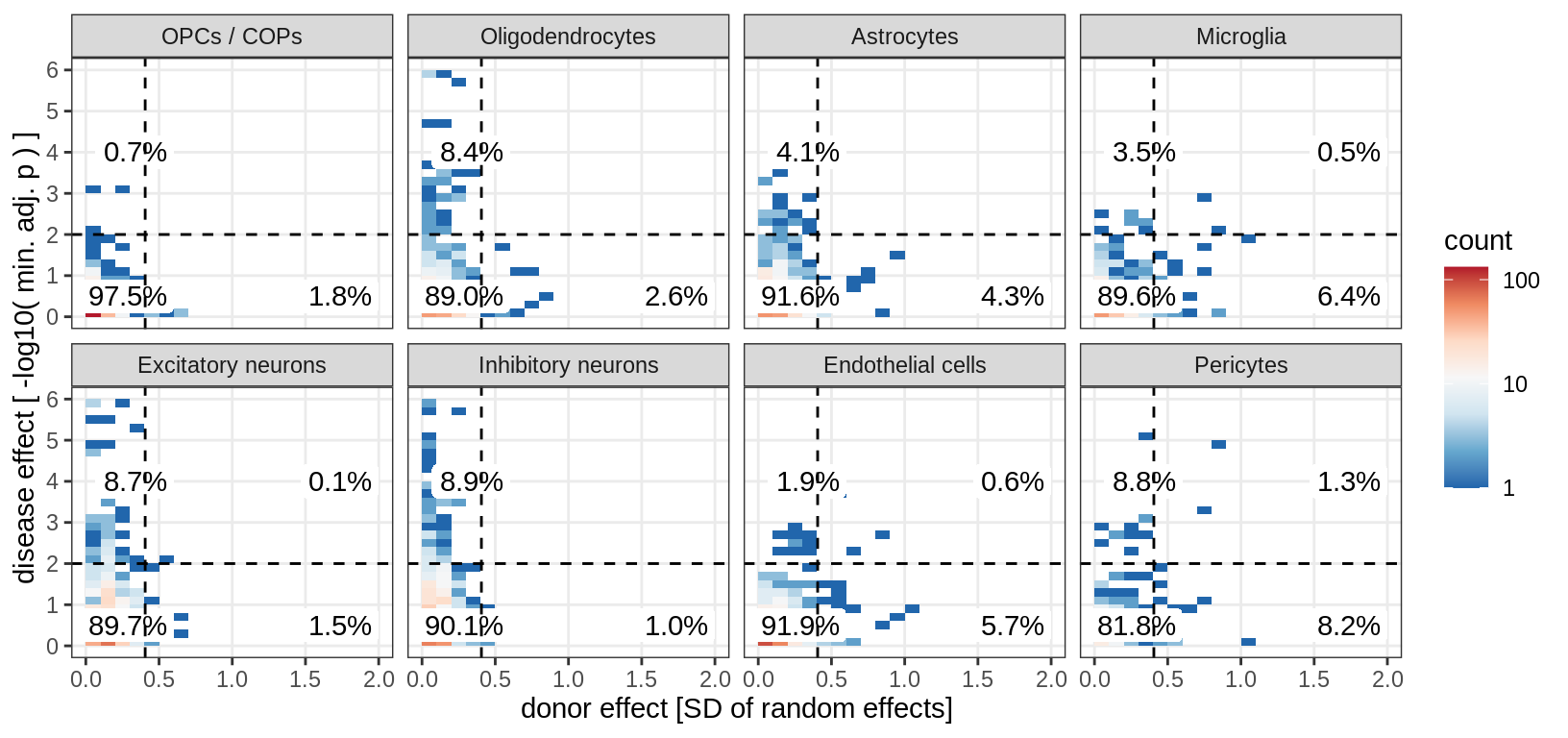
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
muscat results vs LoFs
(plot_muscat_and_sd_vs_lof(res_dt, sd_dt, lof_dt, sel_cl))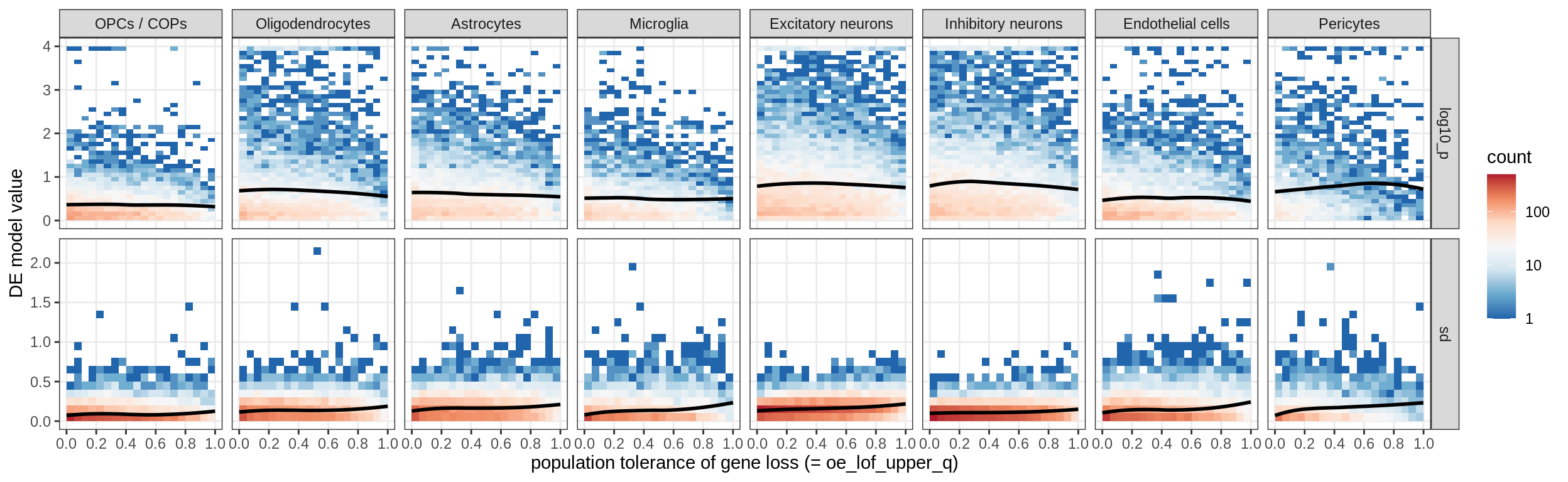
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
Expression heatmap
draw(plot_expression_heatmap_samples(pb, filtered_dt, annots_dt, sel_cl))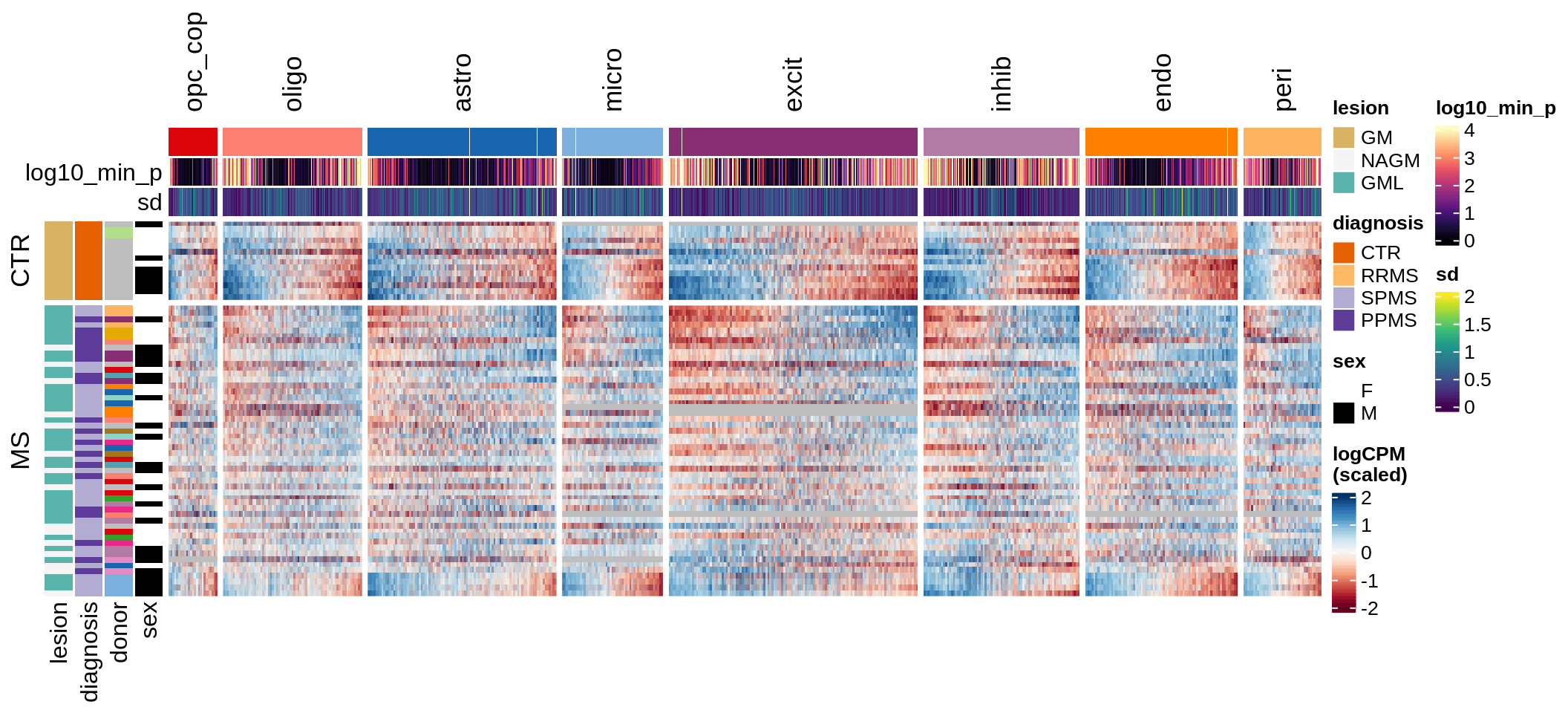
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
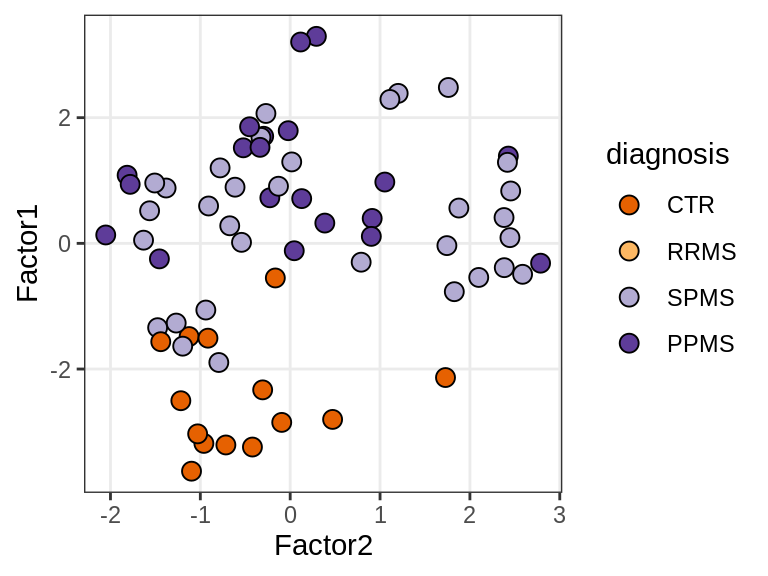
MOFA+ factors - diagnosis
(plot_factors_univariate(model, annots_dt, pb, by = 'diagnosis'))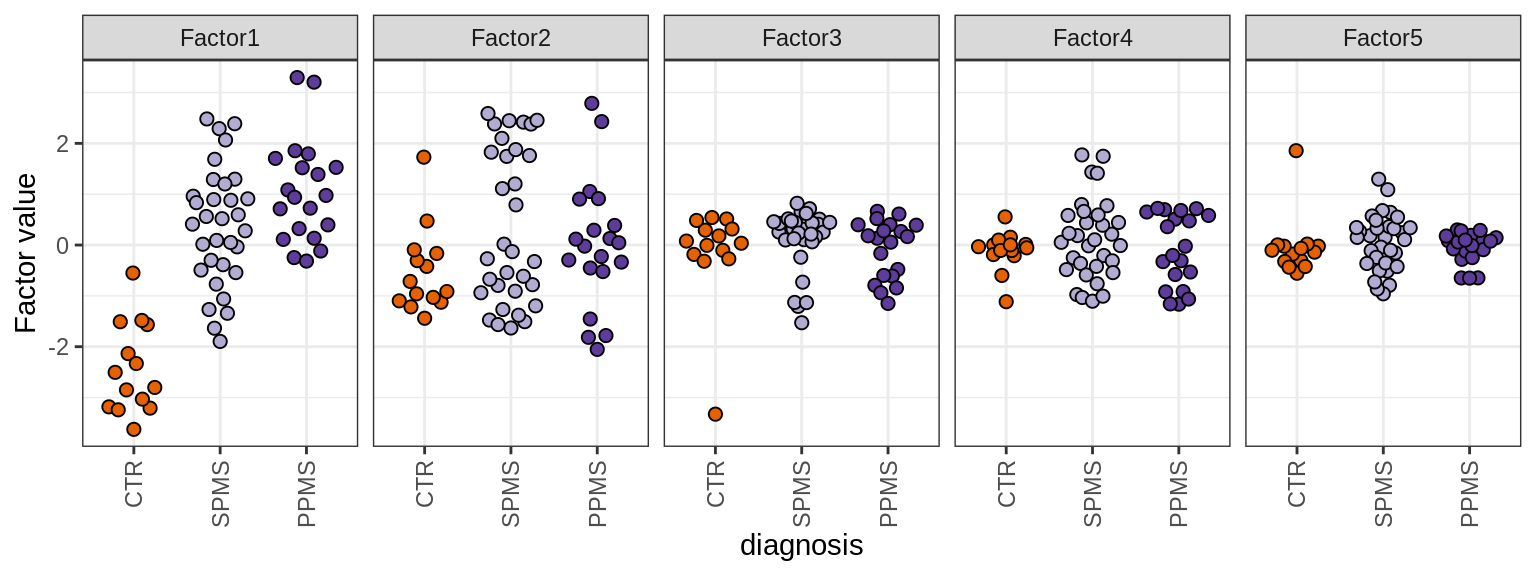
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
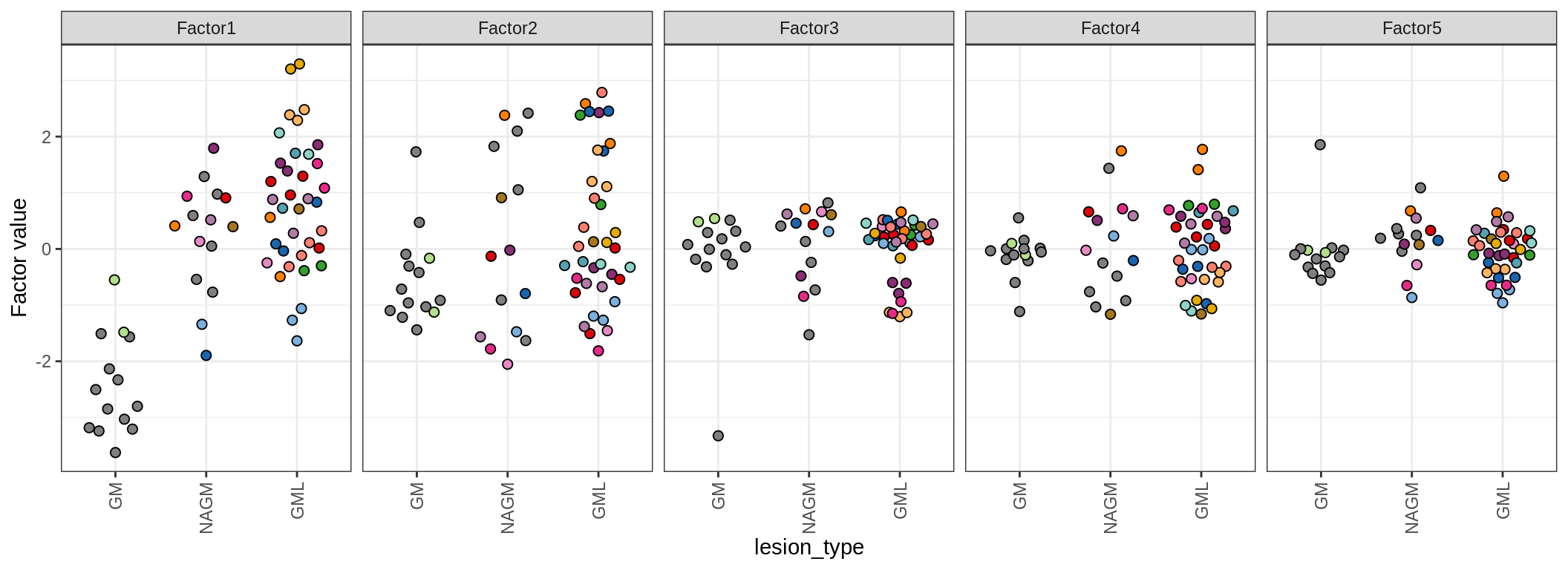
MOFA+ factors - lesions
(plot_factors_univariate(model, annots_dt, pb, by = 'lesion_type'))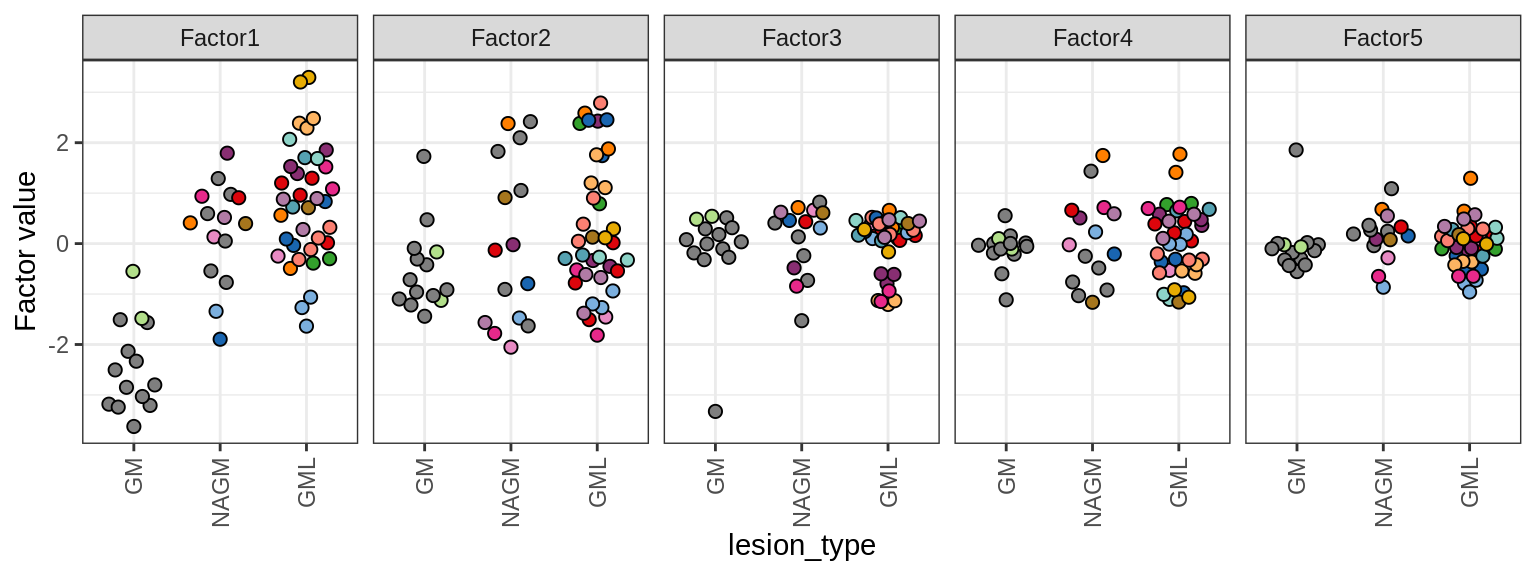
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
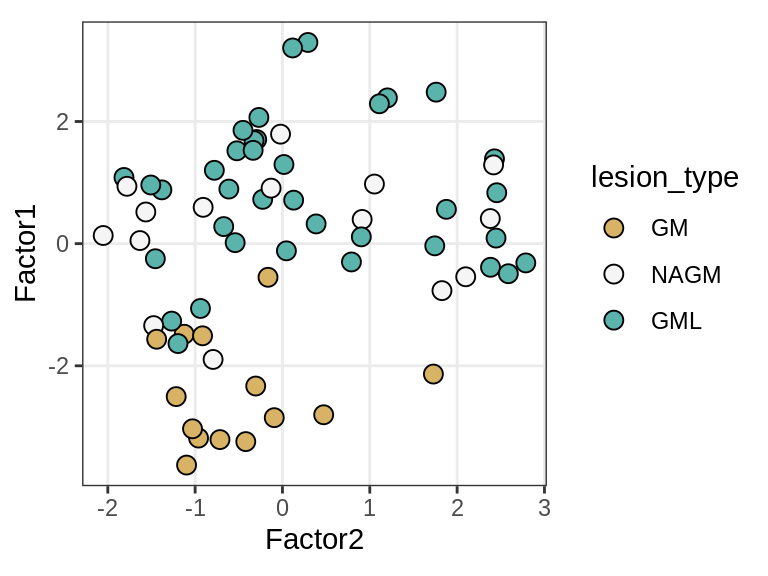
Factor 1 vs Factor 2
for (what in c("diagnosis", "lesion_type", "subject_id")) {
cat('### ', what, '\n', sep = '')
print(plot_factors_pair(model, annots_dt, pb,
f_pair = c("Factor2", "Factor1"), by = what))
cat('\n\n')
}Interactions between factors and model components
(plot_factor_r2s(r2_dt, var_exp_dt))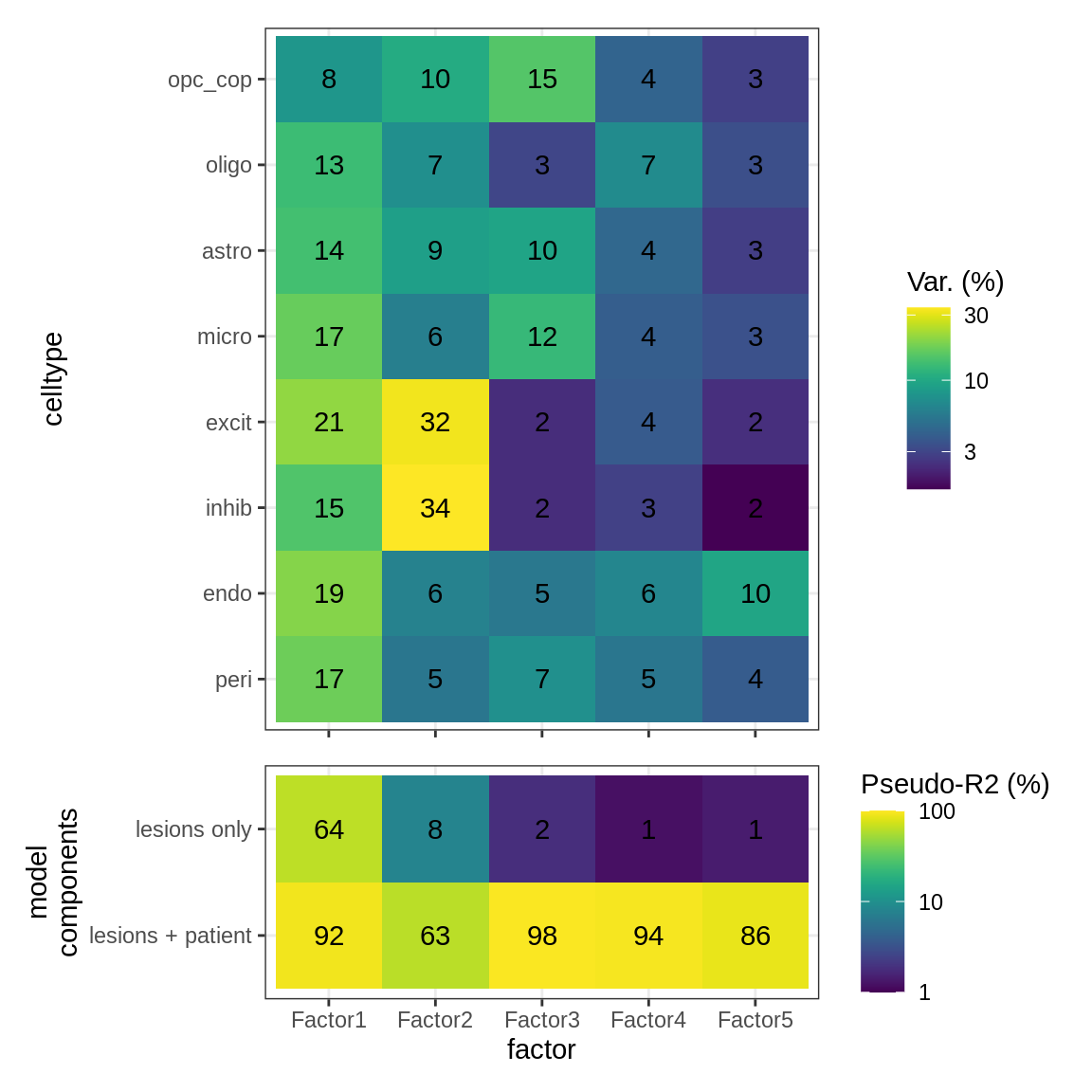
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
GO terms for factors
print(plot_mofa_gsea_dotplot(gsea_list[['go_bp']], labels_dt,
fgsea_cut = fgsea_cut, n_total = 50))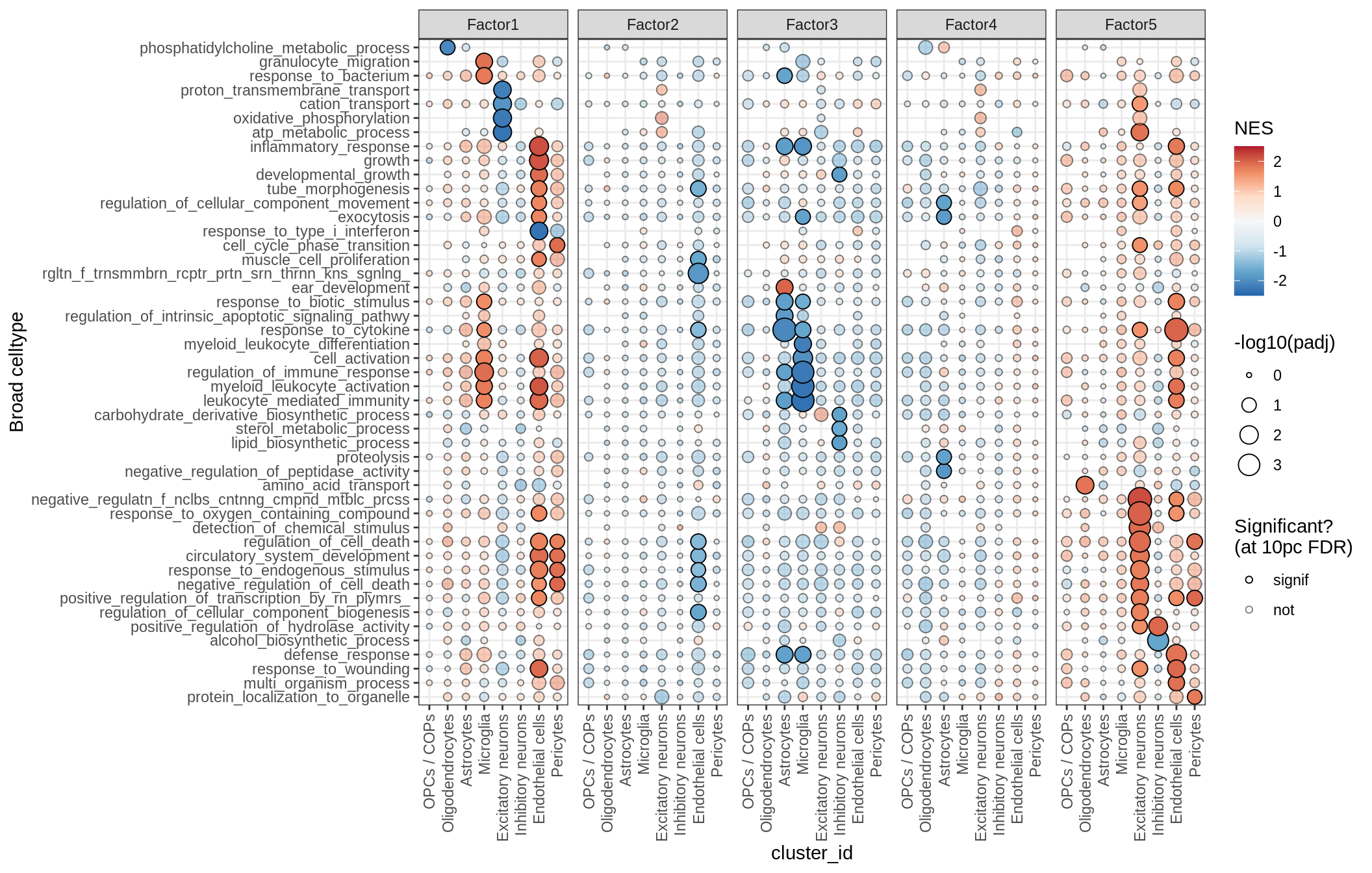
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
Top genes for Factor 1
draw( plot_top_genes_expression(model, pb, annots_dt,
filter_dt, tfs_dt, var_exp_dt, sel_f = 'Factor1',
min_var = 10, min_w = 0.2, n_top = 10, is_regressed = TRUE) )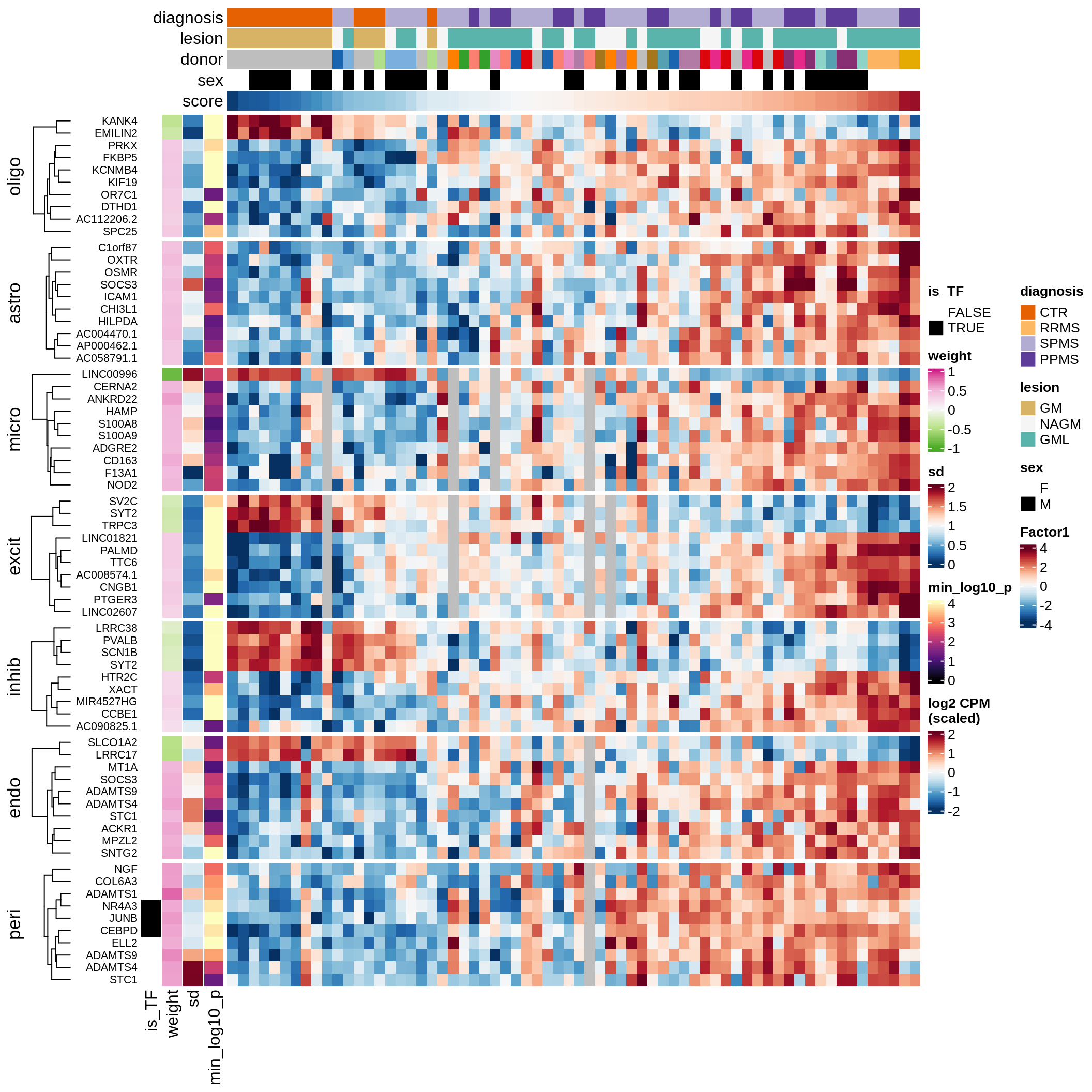
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
Top genes for Factor 2
draw( plot_top_genes_expression(model, pb, annots_dt,
filter_dt, tfs_dt, var_exp_dt, sel_f = 'Factor2',
min_var = 10, min_w = 0.2, n_top = 20, is_regressed = TRUE) )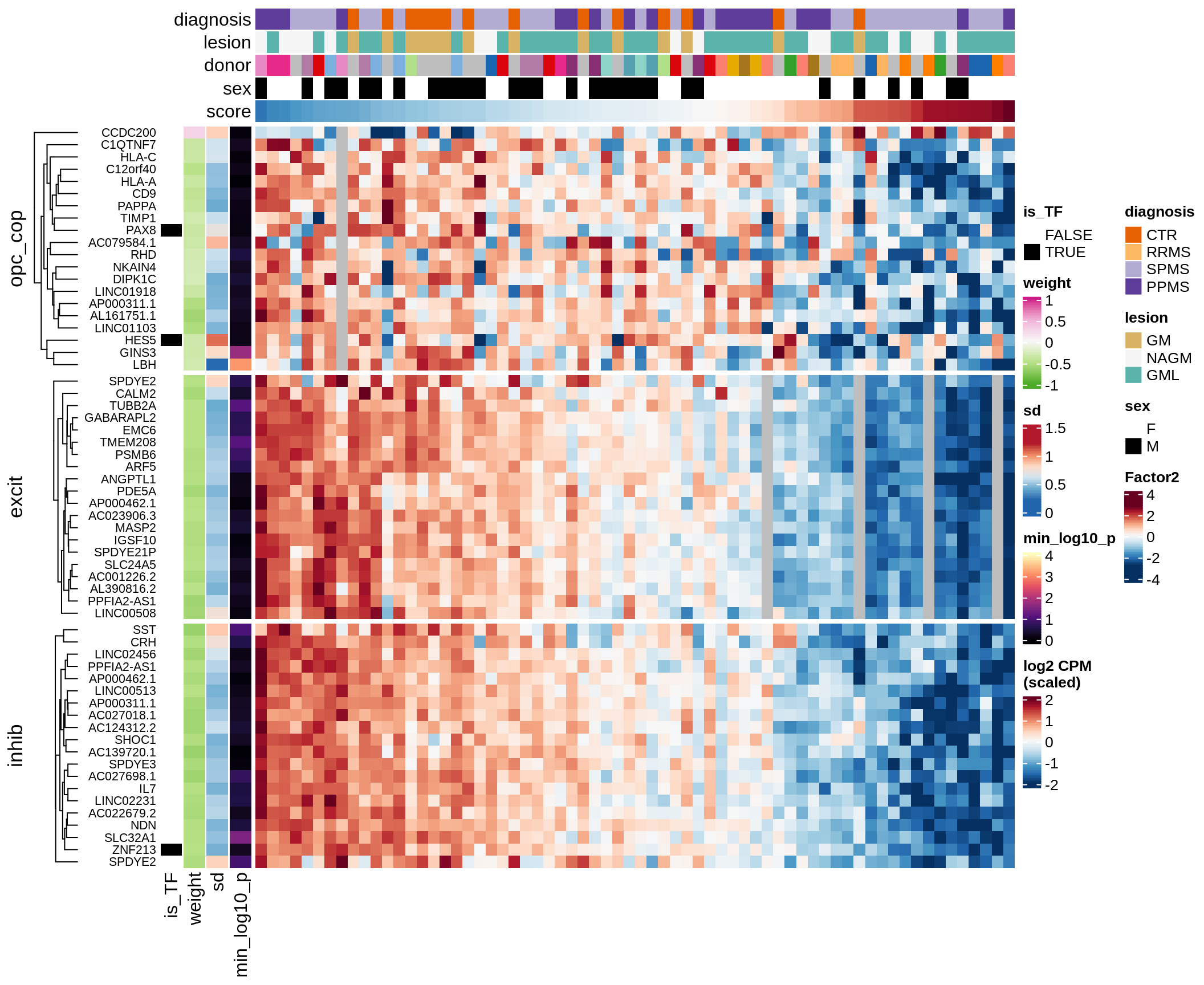
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
Top genes for Factor 3
draw( plot_top_genes_expression(model, pb, annots_dt,
filter_dt, tfs_dt, var_exp_dt, sel_f = 'Factor3',
min_var = 10, min_w = 0.2, n_top = 20, is_regressed = TRUE) )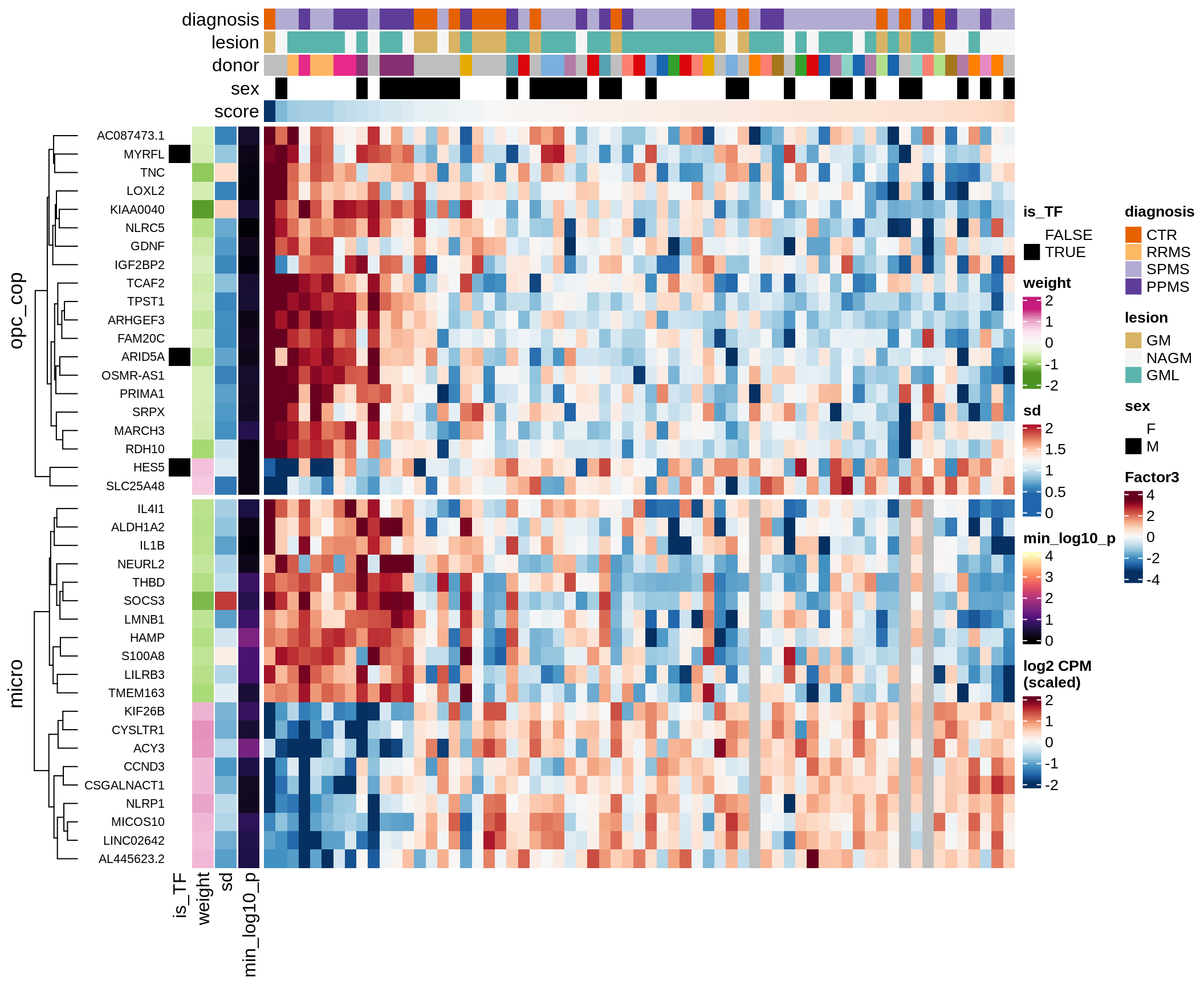
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
Top genes for Factor 4
draw( plot_top_genes_expression(model, pb, annots_dt,
filter_dt, tfs_dt, var_exp_dt, sel_f = 'Factor4',
min_var = 5, min_w = 0.2, n_top = 10, is_regressed = TRUE) )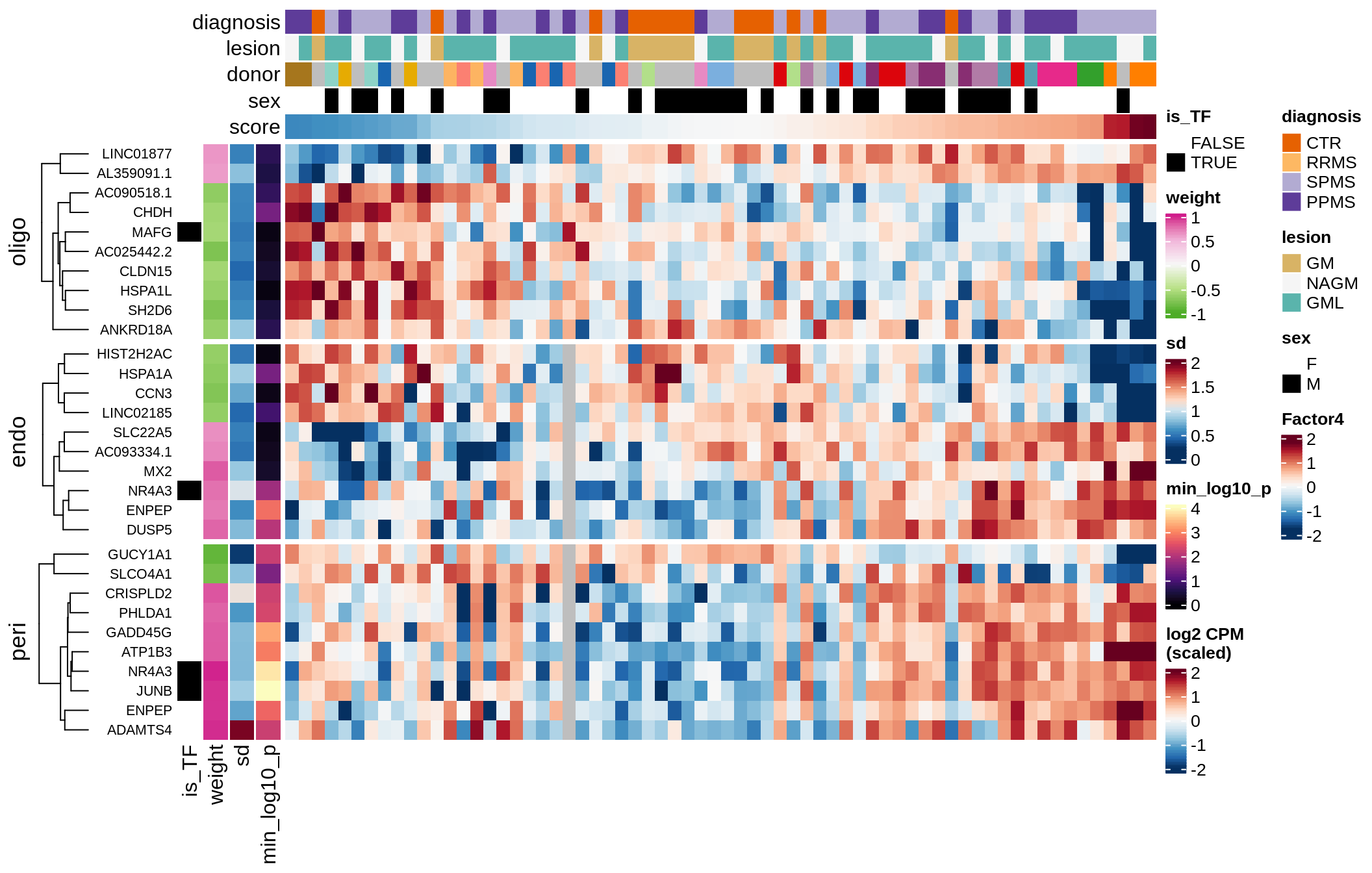
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
Top genes for Factor 5
draw( plot_top_genes_expression(model, pb, annots_dt,
filter_dt, tfs_dt, var_exp_dt, sel_f = 'Factor5',
min_var = 5, min_w = 0.2, n_top = 20, is_regressed = TRUE) )
| Version | Author | Date |
|---|---|---|
| 08fec96 | Macnair | 2021-11-24 |
devtools::session_info()- Session info ---------------------------------------------------------------
setting value
version R version 4.0.5 (2021-03-31)
os CentOS Linux 7 (Core)
system x86_64, linux-gnu
ui X11
language (EN)
collate en_US.UTF-8
ctype C
tz Europe/Zurich
date 2021-11-25
- Packages -------------------------------------------------------------------
package * version date lib
ade4 1.7-18 2021-09-16 [1]
ANCOMBC * 1.0.5 2021-03-09 [1]
annotate 1.68.0 2020-10-27 [1]
AnnotationDbi 1.52.0 2020-10-27 [1]
ape 5.5 2021-04-25 [1]
assertthat * 0.2.1 2019-03-21 [2]
backports 1.2.1 2020-12-09 [2]
basilisk 1.2.1 2020-12-16 [1]
basilisk.utils 1.2.2 2021-01-27 [1]
beachmat 2.6.4 2020-12-20 [1]
beeswarm 0.4.0 2021-06-01 [1]
Biobase * 2.50.0 2020-10-27 [1]
BiocGenerics * 0.36.1 2021-04-16 [1]
BiocManager 1.30.16 2021-06-15 [1]
BiocNeighbors 1.8.2 2020-12-07 [1]
BiocParallel * 1.24.1 2020-11-06 [1]
BiocSingular 1.6.0 2020-10-27 [1]
BiocStyle * 2.18.1 2020-11-24 [1]
biomformat 1.18.0 2020-10-27 [1]
Biostrings 2.58.0 2020-10-27 [1]
bit 4.0.4 2020-08-04 [2]
bit64 4.0.5 2020-08-30 [2]
bitops 1.0-7 2021-04-24 [2]
blme 1.0-5 2021-01-05 [1]
blob 1.2.2 2021-07-23 [2]
boot 1.3-28 2021-05-03 [2]
broom 0.7.9 2021-07-27 [2]
bslib 0.3.1 2021-10-06 [2]
cachem 1.0.6 2021-08-19 [1]
Cairo 1.5-12.2 2020-07-07 [2]
callr 3.7.0 2021-04-20 [2]
caTools 1.18.2 2021-03-28 [2]
cellranger 1.1.0 2016-07-27 [2]
circlize * 0.4.13 2021-06-09 [1]
cli 3.0.1 2021-07-17 [1]
clue 0.3-60 2021-10-11 [1]
cluster 2.1.2 2021-04-17 [2]
codetools 0.2-18 2020-11-04 [2]
colorout * 1.2-2 2021-04-15 [1]
colorRamps 2.3 2012-10-29 [1]
colorspace 2.0-2 2021-06-24 [1]
ComplexHeatmap * 2.6.2 2020-11-12 [1]
corrplot 0.90 2021-06-30 [1]
cowplot 1.1.1 2020-12-30 [2]
crayon 1.4.1 2021-02-08 [2]
data.table * 1.14.2 2021-09-27 [2]
DBI 1.1.1 2021-01-15 [2]
dbplyr 2.1.1 2021-04-06 [2]
DelayedArray 0.16.3 2021-03-24 [1]
DelayedMatrixStats 1.12.3 2021-02-03 [1]
desc 1.4.0 2021-09-28 [1]
DESeq2 1.30.1 2021-02-19 [1]
devtools 2.4.2 2021-06-07 [1]
digest 0.6.28 2021-09-23 [2]
doParallel 1.0.16 2020-10-16 [1]
dplyr * 1.0.7 2021-06-18 [2]
edgeR * 3.32.1 2021-01-14 [1]
ellipsis 0.3.2 2021-04-29 [2]
evaluate 0.14 2019-05-28 [2]
fansi 0.5.0 2021-05-25 [2]
farver 2.1.0 2021-02-28 [2]
fastmap 1.1.0 2021-01-25 [2]
fastmatch 1.1-3 2021-07-23 [1]
fgsea * 1.16.0 2020-10-27 [1]
filelock 1.0.2 2018-10-05 [1]
forcats * 0.5.1 2021-01-27 [2]
foreach 1.5.1 2020-10-15 [2]
fs 1.5.0 2020-07-31 [2]
future 1.22.1 2021-08-25 [2]
future.apply 1.8.1 2021-08-10 [2]
genefilter 1.72.1 2021-01-21 [1]
geneplotter 1.68.0 2020-10-27 [1]
generics 0.1.1 2021-10-25 [2]
GenomeInfoDb * 1.26.7 2021-04-08 [1]
GenomeInfoDbData 1.2.4 2021-04-15 [1]
GenomicAlignments 1.26.0 2020-10-27 [1]
GenomicRanges * 1.42.0 2020-10-27 [1]
GetoptLong 1.0.5 2020-12-15 [1]
ggbeeswarm * 0.6.0 2017-08-07 [1]
ggplot2 * 3.3.5 2021-06-25 [1]
ggrepel * 0.9.1 2021-01-15 [2]
git2r 0.28.0 2021-01-10 [1]
glmmTMB 1.1.2.3 2021-09-20 [1]
GlobalOptions 0.1.2 2020-06-10 [1]
globals 0.14.0 2020-11-22 [2]
glue 1.4.2 2020-08-27 [2]
gplots 3.1.1 2020-11-28 [2]
gridExtra 2.3 2017-09-09 [2]
grr 0.9.5 2016-08-26 [1]
gtable 0.3.0 2019-03-25 [2]
gtools 3.9.2 2021-06-06 [2]
haven 2.4.3 2021-08-04 [2]
HDF5Array 1.18.1 2021-02-04 [1]
here 1.0.1 2020-12-13 [2]
highr 0.9 2021-04-16 [2]
hms 1.1.1 2021-09-26 [1]
htmltools 0.5.2 2021-08-25 [2]
htmlwidgets 1.5.4 2021-09-08 [2]
httpuv 1.6.3 2021-09-09 [2]
httr 1.4.2 2020-07-20 [2]
igraph 1.2.7 2021-10-15 [2]
insight 0.14.5 2021-10-16 [1]
IRanges * 2.24.1 2020-12-12 [1]
irlba 2.3.3 2019-02-05 [2]
iterators 1.0.13 2020-10-15 [2]
janitor 2.1.0 2021-01-05 [1]
jquerylib 0.1.4 2021-04-26 [2]
jsonlite 1.7.2 2020-12-09 [2]
KernSmooth 2.23-20 2021-05-03 [2]
knitr 1.36 2021-09-29 [1]
labeling 0.4.2 2020-10-20 [2]
later 1.3.0 2021-08-18 [2]
lattice 0.20-45 2021-09-22 [2]
lifecycle 1.0.1 2021-09-24 [2]
limma * 3.46.0 2020-10-27 [1]
listenv 0.8.0 2019-12-05 [2]
lme4 1.1-27.1 2021-06-22 [1]
lmerTest 3.1-3 2020-10-23 [1]
locfit 1.5-9.4 2020-03-25 [1]
lubridate 1.8.0 2021-10-07 [2]
magick 2.7.3 2021-08-18 [2]
magrittr * 2.0.1 2020-11-17 [1]
MASS * 7.3-54 2021-05-03 [2]
Matrix * 1.3-4 2021-06-01 [2]
Matrix.utils * 0.9.8 2020-02-26 [1]
MatrixGenerics * 1.2.1 2021-01-30 [1]
matrixStats * 0.61.0 2021-09-17 [1]
memoise 2.0.0 2021-01-26 [1]
mgcv 1.8-38 2021-10-06 [1]
microbiome 1.12.0 2020-10-27 [1]
minqa 1.2.4 2014-10-09 [1]
modelr 0.1.8 2020-05-19 [2]
MOFA2 * 1.0.1 2020-11-03 [1]
multtest 2.46.0 2020-10-27 [1]
munsell 0.5.0 2018-06-12 [2]
muscat * 1.5.1 2021-04-15 [1]
nlme 3.1-153 2021-09-07 [2]
nloptr 1.2.2.2 2020-07-02 [1]
numDeriv 2016.8-1.1 2019-06-06 [2]
parallelly 1.28.1 2021-09-09 [2]
patchwork * 1.1.1 2020-12-17 [2]
pbkrtest 0.5.1 2021-03-09 [1]
performance * 0.8.0 2021-10-01 [1]
permute 0.9-5 2019-03-12 [1]
pheatmap 1.0.12 2019-01-04 [1]
phyloseq * 1.34.0 2020-10-27 [1]
pillar 1.6.4 2021-10-18 [1]
pkgbuild 1.2.0 2020-12-15 [1]
pkgconfig 2.0.3 2019-09-22 [2]
pkgload 1.2.3 2021-10-13 [2]
plyr 1.8.6 2020-03-03 [2]
png 0.1-7 2013-12-03 [2]
prettyunits 1.1.1 2020-01-24 [2]
processx 3.5.2 2021-04-30 [2]
progress 1.2.2 2019-05-16 [2]
promises 1.2.0.1 2021-02-11 [2]
ps 1.6.0 2021-02-28 [2]
purrr * 0.3.4 2020-04-17 [2]
R.methodsS3 1.8.1 2020-08-26 [1]
R.oo 1.24.0 2020-08-26 [1]
R.utils 2.11.0 2021-09-26 [1]
R6 2.5.1 2021-08-19 [2]
rappdirs 0.3.3 2021-01-31 [2]
rbibutils 2.2.4 2021-10-11 [1]
RColorBrewer * 1.1-2 2014-12-07 [2]
Rcpp 1.0.7 2021-07-07 [1]
RCurl 1.98-1.5 2021-09-17 [1]
Rdpack 2.1.2 2021-06-01 [1]
readr * 2.0.2 2021-09-27 [2]
readxl * 1.3.1 2019-03-13 [2]
registry 0.5-1 2019-03-05 [1]
remotes 2.4.1 2021-09-29 [1]
reprex 2.0.1 2021-08-05 [2]
reshape2 * 1.4.4 2020-04-09 [2]
reticulate * 1.22 2021-09-17 [2]
rgl * 0.107.14 2021-08-21 [1]
rhdf5 2.34.0 2020-10-27 [1]
rhdf5filters 1.2.1 2021-05-03 [1]
Rhdf5lib 1.12.1 2021-01-26 [1]
rjson 0.2.20 2018-06-08 [1]
rlang 0.4.12 2021-10-18 [2]
rmarkdown * 2.11 2021-09-14 [1]
rprojroot 2.0.2 2020-11-15 [2]
Rsamtools 2.6.0 2020-10-27 [1]
RSQLite 2.2.8 2021-08-21 [1]
rstudioapi 0.13 2020-11-12 [2]
rsvd 1.0.5 2021-04-16 [1]
rtracklayer * 1.50.0 2020-10-27 [1]
Rtsne 0.15 2018-11-10 [2]
rvest 1.0.2 2021-10-16 [2]
S4Vectors * 0.28.1 2020-12-09 [1]
sass 0.4.0 2021-05-12 [2]
scales * 1.1.1 2020-05-11 [2]
scater * 1.18.6 2021-02-26 [1]
sctransform 0.3.2 2020-12-16 [2]
scuttle 1.0.4 2020-12-17 [1]
seriation * 1.3.1 2021-10-16 [1]
sessioninfo 1.1.1 2018-11-05 [1]
shape 1.4.6 2021-05-19 [1]
SingleCellExperiment * 1.12.0 2020-10-27 [1]
snakecase 0.11.0 2019-05-25 [1]
sparseMatrixStats 1.2.1 2021-02-02 [1]
stringi 1.7.4 2021-08-25 [1]
stringr * 1.4.0 2019-02-10 [2]
SummarizedExperiment * 1.20.0 2020-10-27 [1]
survival 3.2-13 2021-08-24 [2]
testthat 3.1.0 2021-10-04 [2]
tibble * 3.1.5 2021-09-30 [1]
tictoc * 1.0.1 2021-04-19 [1]
tidyr * 1.1.4 2021-09-27 [2]
tidyselect 1.1.1 2021-04-30 [2]
tidyverse * 1.3.1 2021-04-15 [2]
TMB 1.7.22 2021-09-28 [1]
TSP 1.1-11 2021-10-06 [1]
tzdb 0.1.2 2021-07-20 [2]
UpSetR * 1.4.0 2019-05-22 [1]
usethis 2.1.2 2021-10-25 [1]
utf8 1.2.2 2021-07-24 [1]
uwot 0.1.10 2020-12-15 [2]
variancePartition 1.20.0 2020-10-27 [1]
vctrs 0.3.8 2021-04-29 [2]
vegan 2.5-7 2020-11-28 [1]
vipor 0.4.5 2017-03-22 [1]
viridis * 0.6.2 2021-10-13 [1]
viridisLite * 0.4.0 2021-04-13 [1]
whisker 0.4 2019-08-28 [1]
withr 2.4.2 2021-04-18 [2]
workflowr * 1.6.2 2020-04-30 [1]
writexl * 1.4.0 2021-04-20 [1]
xfun 0.27 2021-10-18 [1]
XML 3.99-0.8 2021-09-17 [1]
xml2 1.3.2 2020-04-23 [2]
xtable 1.8-4 2019-04-21 [2]
XVector 0.30.0 2020-10-27 [1]
yaml 2.2.1 2020-02-01 [2]
zlibbioc 1.36.0 2020-10-27 [1]
source
CRAN (R 4.0.5)
Bioconductor
Bioconductor
Bioconductor
CRAN (R 4.0.3)
CRAN (R 4.0.0)
CRAN (R 4.0.3)
Bioconductor
Bioconductor
Bioconductor
CRAN (R 4.0.3)
Bioconductor
Bioconductor
CRAN (R 4.0.3)
Bioconductor
Bioconductor
Bioconductor
Bioconductor
Bioconductor
Bioconductor
CRAN (R 4.0.2)
CRAN (R 4.0.2)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.2)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.0)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
Github (jalvesaq/colorout@79931fd)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
Bioconductor
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
Bioconductor
Bioconductor
CRAN (R 4.0.5)
Bioconductor
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
Bioconductor
CRAN (R 4.0.3)
CRAN (R 4.0.0)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
Bioconductor
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.2)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
Bioconductor
Bioconductor
CRAN (R 4.0.5)
Bioconductor
Bioconductor
Bioconductor
Bioconductor
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.0)
CRAN (R 4.0.3)
CRAN (R 4.0.0)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
Bioconductor
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.2)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
Bioconductor
CRAN (R 4.0.0)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
Bioconductor
CRAN (R 4.0.0)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
Bioconductor
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
Bioconductor
CRAN (R 4.0.3)
CRAN (R 4.0.0)
Bioconductor
Bioconductor
CRAN (R 4.0.0)
Github (HelenaLC/muscat@c939663)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.2)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
Bioconductor
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.0)
CRAN (R 4.0.5)
CRAN (R 4.0.0)
CRAN (R 4.0.0)
CRAN (R 4.0.0)
CRAN (R 4.0.3)
CRAN (R 4.0.0)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.0)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.0)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.0)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.0)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
Bioconductor
Bioconductor
Bioconductor
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
Bioconductor
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
Bioconductor
CRAN (R 4.0.0)
CRAN (R 4.0.5)
Bioconductor
CRAN (R 4.0.3)
CRAN (R 4.0.0)
Bioconductor
CRAN (R 4.0.3)
Bioconductor
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.1)
Bioconductor
CRAN (R 4.0.3)
Bioconductor
CRAN (R 4.0.5)
CRAN (R 4.0.0)
Bioconductor
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
Bioconductor
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.1)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.3)
CRAN (R 4.0.5)
CRAN (R 4.0.5)
CRAN (R 4.0.0)
CRAN (R 4.0.0)
Bioconductor
CRAN (R 4.0.3)
Bioconductor
[1] /pstore/home/macnairw/lib/conda_r3.12
[2] /pstore/home/macnairw/.conda/envs/r_4.0.3/lib/R/library
sessionInfo()R version 4.0.5 (2021-03-31)
Platform: x86_64-conda-linux-gnu (64-bit)
Running under: CentOS Linux 7 (Core)
Matrix products: default
BLAS/LAPACK: /pstore/home/macnairw/.conda/envs/r_4.0.3/lib/libopenblasp-r0.3.12.so
locale:
[1] LC_CTYPE=C LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] grid parallel stats4 stats graphics grDevices utils
[8] datasets methods base
other attached packages:
[1] rgl_0.107.14 MOFA2_1.0.1
[3] rmarkdown_2.11 writexl_1.4.0
[5] ComplexHeatmap_2.6.2 fgsea_1.16.0
[7] tictoc_1.0.1 performance_0.8.0
[9] edgeR_3.32.1 limma_3.46.0
[11] reshape2_1.4.4 scater_1.18.6
[13] Matrix.utils_0.9.8 Matrix_1.3-4
[15] SingleCellExperiment_1.12.0 SummarizedExperiment_1.20.0
[17] Biobase_2.50.0 MatrixGenerics_1.2.1
[19] matrixStats_0.61.0 seriation_1.3.1
[21] UpSetR_1.4.0 BiocParallel_1.24.1
[23] muscat_1.5.1 dplyr_1.0.7
[25] readr_2.0.2 tidyr_1.1.4
[27] tibble_3.1.5 tidyverse_1.3.1
[29] rtracklayer_1.50.0 GenomicRanges_1.42.0
[31] GenomeInfoDb_1.26.7 IRanges_2.24.1
[33] S4Vectors_0.28.1 BiocGenerics_0.36.1
[35] ggbeeswarm_0.6.0 ggrepel_0.9.1
[37] reticulate_1.22 MASS_7.3-54
[39] phyloseq_1.34.0 ANCOMBC_1.0.5
[41] purrr_0.3.4 patchwork_1.1.1
[43] readxl_1.3.1 forcats_0.5.1
[45] ggplot2_3.3.5 scales_1.1.1
[47] viridis_0.6.2 viridisLite_0.4.0
[49] assertthat_0.2.1 stringr_1.4.0
[51] data.table_1.14.2 magrittr_2.0.1
[53] circlize_0.4.13 RColorBrewer_1.1-2
[55] BiocStyle_2.18.1 colorout_1.2-2
[57] workflowr_1.6.2
loaded via a namespace (and not attached):
[1] rappdirs_0.3.3 R.methodsS3_1.8.1
[3] bit64_4.0.5 knitr_1.36
[5] R.utils_2.11.0 irlba_2.3.3
[7] DelayedArray_0.16.3 RCurl_1.98-1.5
[9] doParallel_1.0.16 generics_0.1.1
[11] callr_3.7.0 cowplot_1.1.1
[13] microbiome_1.12.0 usethis_2.1.2
[15] RSQLite_2.2.8 future_1.22.1
[17] bit_4.0.4 tzdb_0.1.2
[19] xml2_1.3.2 lubridate_1.8.0
[21] httpuv_1.6.3 xfun_0.27
[23] hms_1.1.1 jquerylib_0.1.4
[25] TSP_1.1-11 evaluate_0.14
[27] promises_1.2.0.1 fansi_0.5.0
[29] progress_1.2.2 caTools_1.18.2
[31] dbplyr_2.1.1 htmlwidgets_1.5.4
[33] igraph_1.2.7 DBI_1.1.1
[35] geneplotter_1.68.0 ellipsis_0.3.2
[37] corrplot_0.90 backports_1.2.1
[39] insight_0.14.5 permute_0.9-5
[41] annotate_1.68.0 sparseMatrixStats_1.2.1
[43] vctrs_0.3.8 remotes_2.4.1
[45] here_1.0.1 Cairo_1.5-12.2
[47] cachem_1.0.6 withr_2.4.2
[49] grr_0.9.5 sctransform_0.3.2
[51] vegan_2.5-7 GenomicAlignments_1.26.0
[53] prettyunits_1.1.1 cluster_2.1.2
[55] ape_5.5 crayon_1.4.1
[57] basilisk.utils_1.2.2 genefilter_1.72.1
[59] labeling_0.4.2 pkgconfig_2.0.3
[61] pkgload_1.2.3 nlme_3.1-153
[63] vipor_0.4.5 devtools_2.4.2
[65] blme_1.0-5 rlang_0.4.12
[67] globals_0.14.0 lifecycle_1.0.1
[69] filelock_1.0.2 registry_0.5-1
[71] modelr_0.1.8 rsvd_1.0.5
[73] cellranger_1.1.0 rprojroot_2.0.2
[75] Rhdf5lib_1.12.1 boot_1.3-28
[77] reprex_2.0.1 beeswarm_0.4.0
[79] processx_3.5.2 pheatmap_1.0.12
[81] whisker_0.4 GlobalOptions_0.1.2
[83] png_0.1-7 rjson_0.2.20
[85] bitops_1.0-7 R.oo_1.24.0
[87] KernSmooth_2.23-20 rhdf5filters_1.2.1
[89] Biostrings_2.58.0 blob_1.2.2
[91] DelayedMatrixStats_1.12.3 shape_1.4.6
[93] parallelly_1.28.1 beachmat_2.6.4
[95] memoise_2.0.0 plyr_1.8.6
[97] gplots_3.1.1 zlibbioc_1.36.0
[99] compiler_4.0.5 clue_0.3-60
[101] lme4_1.1-27.1 DESeq2_1.30.1
[103] snakecase_0.11.0 Rsamtools_2.6.0
[105] cli_3.0.1 ade4_1.7-18
[107] XVector_0.30.0 lmerTest_3.1-3
[109] listenv_0.8.0 ps_1.6.0
[111] TMB_1.7.22 mgcv_1.8-38
[113] tidyselect_1.1.1 stringi_1.7.4
[115] highr_0.9 yaml_2.2.1
[117] BiocSingular_1.6.0 locfit_1.5-9.4
[119] sass_0.4.0 fastmatch_1.1-3
[121] tools_4.0.5 future.apply_1.8.1
[123] rstudioapi_0.13 foreach_1.5.1
[125] git2r_0.28.0 janitor_2.1.0
[127] gridExtra_2.3 farver_2.1.0
[129] Rtsne_0.15 digest_0.6.28
[131] BiocManager_1.30.16 Rcpp_1.0.7
[133] broom_0.7.9 scuttle_1.0.4
[135] later_1.3.0 httr_1.4.2
[137] AnnotationDbi_1.52.0 Rdpack_2.1.2
[139] colorspace_2.0-2 rvest_1.0.2
[141] XML_3.99-0.8 fs_1.5.0
[143] splines_4.0.5 uwot_0.1.10
[145] basilisk_1.2.1 multtest_2.46.0
[147] sessioninfo_1.1.1 xtable_1.8-4
[149] jsonlite_1.7.2 nloptr_1.2.2.2
[151] testthat_3.1.0 R6_2.5.1
[153] pillar_1.6.4 htmltools_0.5.2
[155] glue_1.4.2 fastmap_1.1.0
[157] minqa_1.2.4 BiocNeighbors_1.8.2
[159] codetools_0.2-18 pkgbuild_1.2.0
[161] utf8_1.2.2 lattice_0.20-45
[163] bslib_0.3.1 numDeriv_2016.8-1.1
[165] pbkrtest_0.5.1 colorRamps_2.3
[167] gtools_3.9.2 magick_2.7.3
[169] survival_3.2-13 glmmTMB_1.1.2.3
[171] desc_1.4.0 biomformat_1.18.0
[173] munsell_0.5.0 GetoptLong_1.0.5
[175] rhdf5_2.34.0 GenomeInfoDbData_1.2.4
[177] iterators_1.0.13 HDF5Array_1.18.1
[179] variancePartition_1.20.0 haven_2.4.3
[181] gtable_0.3.0 rbibutils_2.2.4